In 2019, Vinmec High-Tech Center successfully established a quality management system according to the ISO 15189:2012 standard and was accredited by the Vietnam Accreditation Office (BOA) with the code VILAS MED 103.
Today, quality is always a top priority for all products and services provided by manufacturing and business establishments. In the field of medical testing, the quality of tests is reflected in ensuring the accuracy, reliability, and timeliness of test results, which play a crucial role in diagnostic decisions, treatment, and prognosis by clinical doctors for patients or clients.
ThS. Nguyễn Thị Ngọc Hà, Quality Manager, DGCLSP Group, High-Tech Center
ThS. Nguyễn Đắc Tú, Head of Product Quality Evaluation Group, High-Tech Center
Nowadays, quality is a top priority for all products and services provided by manufacturing and business establishments. In the field of medical testing, the quality of tests is reflected in ensuring the accuracy, reliability, and timeliness of test results, which play a crucial role in diagnostic decisions, treatment, and prognosis by clinical doctors for patients or clients. To ensure quality, laboratories need to build a quality management system based on national and international standards. One of the internationally recognized standards widely applied in laboratories in Vietnam and other countries around the world is the ISO 15189:2012 standard.
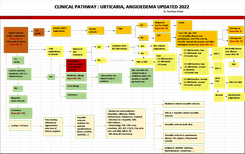
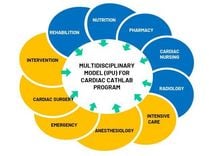
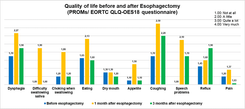
ISO 15189:2012 is a standard that outlines the requirements for the competence and quality of medical laboratories, developed by the International Organization for Standardization (ISO). This standard is based on the requirements of ISO/IEC 17025 and ISO 9001, with additional specific requirements to ensure quality in the field of medical testing. The standard includes 15 management requirements and 10 technical requirements related to ensuring the quality of tests. The management requirements ensure that the laboratory sets up an organizational structure, policies, and quality objectives that are appropriate to the actual situation, establishes mechanisms to implement and monitor compliance with the system, and seeks opportunities for quality improvement. Meanwhile, the technical requirements focus on ensuring quality in the testing process, including evaluating staff competence, controlling equipment, environmental conditions, chemicals, and materials, and monitoring the pre-analytical, analytical, and post-analytical processes.
In 2019, Vinmec High-Tech Center successfully developed a quality management system according to ISO 15189:2012 and was accredited by the Vietnam Accreditation Office (BOA) with the code VILAS MED 103. This certification is proof of the center's capability in performing tests. Notably, Vinmec High-Tech Center was the first unit to be accredited by BOA for the capability to perform Hematopoietic Stem Cell CD34+ Counting tests and is the only unit recognized for the capability to perform tests on T, B, NK immune cell counts and endotoxin bacterial tests according to ISO 15189:2012. To this day, the center continues to maintain and continuously improve its quality to ensure the provision of accurate, reliable, and timely test results that meet the healthcare needs of patients at Vinmec.