I. BACKGROUND
Chronic urticaria (CU) is characterized by itching, hives, and/or angioedema for at least 6 weeks. The estimated prevalence of CU varies from 0.1% in North America to 1.4% in Asia.CU can present with daily or almost daily signs and symptoms or an intermittent/recurrent course.
CU has negative impacts on the quality of life (QoL), reduces work productivity and activities of daily living. More than 20% of CU patients reported ≥ 1 hour per week of missed work; productivity impairment has been measured at 27%. Beside that, significant healthcare resources and costs have been incurred to treat CU.
In the practical process of examination and treatment, we recognize that despite visiting numerous clinics and seeing different physicians of different specialties, CU patients often end up with the uncontrolled disease. Some patients have even reached out for non-scientifically and medically approved therapies, which lead to adverse consequences. Therefore, it is necessary to have centers that are competent to support the patients with CU, raise their knowledge and reduce side effects by providing appropriate treatment and care.
The UCARE program is an initiative by the Global Allergy and Asthma Excellence Network (GA2LEN) to increase the knowledge of urticaria by pursuing research, advocating for awareness, and accrediting an interactive network of centers of reference and excellence in urticaria management. Now, there are 164 certified centers in 45 countries in 6 regions worldwide.
In Vietnam, there have been no UCARE certified-centers. Vinmec Times City International Hospital has taken the lead in establishing an urticaria center of reference and excellence following the Integrated Practice Unit (IPU) model in accordance with the quality standards of GA2LEN. The program aims to improve the effectiveness of chronic urticaria management, thereby helping to enhance the quality of life of the patients.
II. TARGETS
By applying the UCARE standards, Vinmec Times City International Hospital aims to pursue the following goals:
- To screen for comorbidities with chronic urticaria such as autoimmune disease, allergic diseases, etc. for all urticaria patients presented to Vinmec Times City Allergy and Immunology Clinics (measured via the rate of compliance with Clinical Pathway).
- To improve the urticaria control test (UCT) score after 4 weeks of treatment to at least 12 (well-controlled).
- To improve quality of life of urticaria patients after 4 weeks of treatment (measured via the chronic urticaria – quality of life CU-Q2oL score).
- ≥ 70% of chronic urticaria patients with good disease control scores after 1 year of treatment.
- To reduce the rate of complications associated with side effects of inappropriate urticaria treatment such as corticosteroids, herbal medicine of unknown origin, etc.
- To reduce opportunity costs caused by the burden of urticaria such as: reduce working hours; examination cost,...
III. IMPROVEMENT SOLUTIONS
PHASE 1. PROGRAM DEVELOPMENT (Q1 2023)
Developing documents of the program:
- A Clinical Pathway in accordance with the UCARE standards.
- A compliance checklist to assess compliance with the Clinical Pathway.
- Patient education materials with a focus on CU.
Standardizing clinical practice:
- Organizing training for physicians and nurses within the IPU, aiming at standardizing their knowledge and practice in accordance with the Clinical Pathway developed based on the UCARE standards.
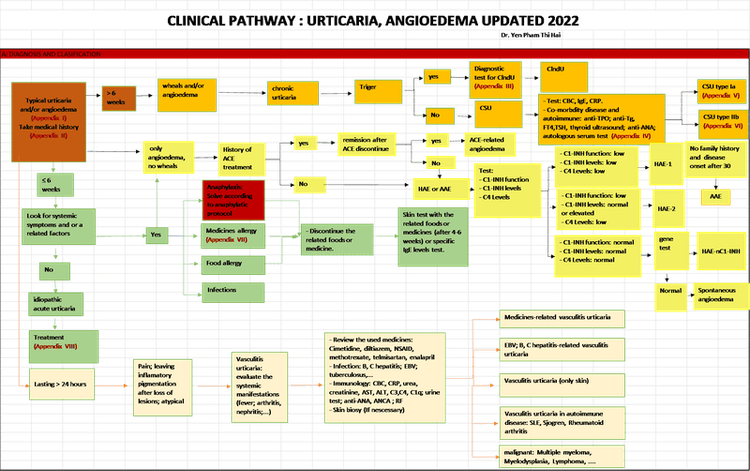
PHASE 2. IMPLEMENTATION (FROM Q2 2023 – PRESENT)
On a monthly basis, all urticaria cases were reviewed and scored for the compliance rate with the Clinical Pathway, assessment of UCT score improvement, and administration of the CU-Q2oL quality of life questionnaire. Monthly review and feedback had contributed to and accelerated the integration of UCARE standards into daily practice of the clinicians.
To further ensure the effectiveness and sustainability of the program, we had organized an urticaria club to provide the most up-to-date knowledge about the condition on a regular basis. This also created a forum for the patients to discuss and share their experience.
PHASE 3: SUBMISSION
On the foundation of the Urticaria IPU, we plan to submit our application to the GA2LEN board for UCARE accreditation in June 2024.
IV. RESULTS
1. The number of urticaria patients who examined in Vinmec Times City Hospital
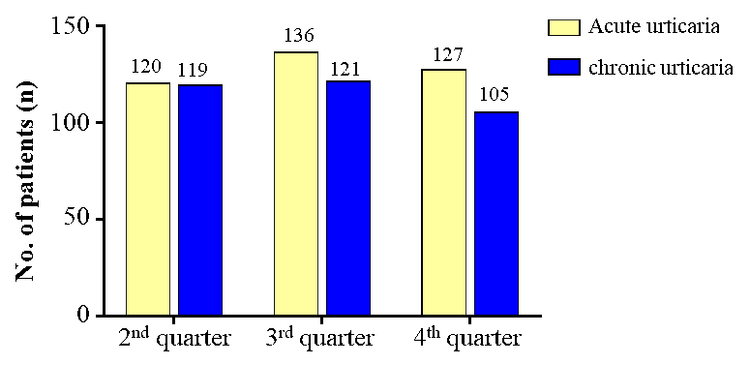
2.Compliance with the urticaria Clinical pathway
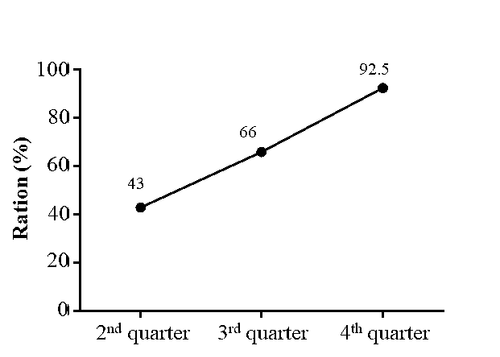
Starting at the rate of 43% in the first quarter of implementation, the rate gradually increased and reached 92.5% by the end of 2023. In order to achieve the result, providing continuous training, monthly review and feedback to the clinicians was the approach chosen.
Accurate diagnosis, disease classification, severity grading, and comorbidities screening such as autoimmune diseases, allergic diseases, etc. are essential components required in the Clinical Pathway. By monitoring the compliance rate with the Clinical Pathway, we were able to effectively promote the standardization process, as well as to reduce variations in practice of the clinicians.
3.Improvement of UCT score after 4 weeks of treatment.
UCT (urticaria control test) measures the control level of urticaria activity. Through the improvement of UCT score, the clinicians can evaluate treatment effectiveness and decide appropriate treatment strategy.
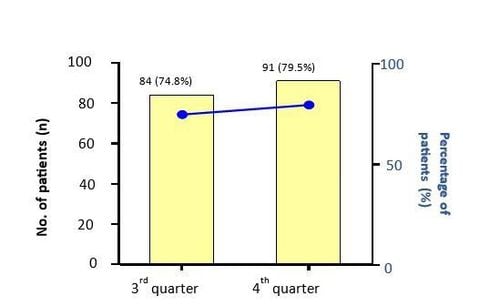
During the 3rd and the 4th quarter of 2023, the proportion of chronic urticaria patients having improvement in UCT score after 4 weeks of treatment remained above 70%.
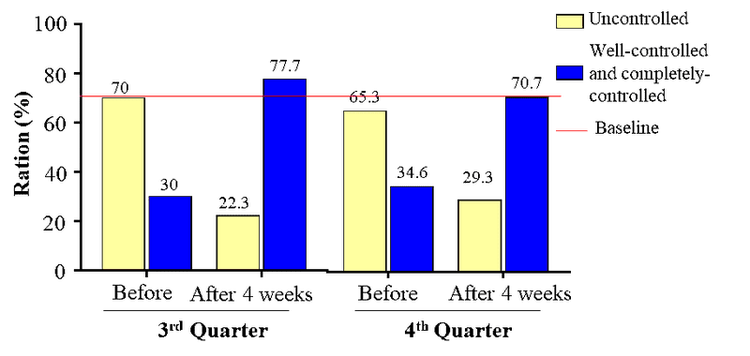
The Quality of life survey showed that after 4 weeks of treatment, averagely, 64.45% of the patients reported an improvement, while approximately 29.75% of those had a complete improvement. This was consistent with the result of UCT score reassessment.
V. CONCLUSION
Vinmec Healthcare system’s urticaria management program, in accordance with the reputable standards of GA2LEN has enhanced the quality of management and care for urticaria patients, especially chronic urticaria. The improvement will be shown by the following outcomes and some, as noted by an asterisk, have already been achieved:
- Improving the urticaria control test (UCT) score after 4 weeks of treatment.*
- Improving chronic urticaria – quality of life (CU-Q2oL) score after 4 weeks of treatment.*
- Screening for comorbidities with chronic urticaria such as autoimmune disease, allergic diseases, etc*
And these means that:
- Reducing the rate of complications associated with side effects of urticaria treatment, such as corticosteroids, herbal medicine of unknown origin, etc.
- Reducing opportunity costs caused by the burden of urticaria such as: reduce working hours; examination costs, ...
Moreover, we aim for a minimum of 70% of chronic urticaria patients to achieve good disease control score at the 1 year mark. We do believe that all of the efforts and treatment outcomes can lay a solid foundation to help Vinmec Times City become the first UCARE-certified center in Vietnam.
REFERENCES
- Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393-1414. doi:10.1111/all.13397
- Fricke J, Ávila G, Keller T, et al. Prevalence of chronic urticaria in children and adults across the globe: Systematic review with meta-analysis. Allergy. 2020;75(2):423-432. doi:10.1111/all.14037
- Maurer M, Abuzakouk M, Bérard F, et al. The burden of chronic spontaneous urticaria is substantial: Real-world evidence from ASSURE-CSU. Allergy. 2017;72(12):2005-2016. doi:10.1111/all.13209
- Thøgersen M. Home. UCARE. Accessed January 29, 2024. https://ga2len-ucare.com/
- Maurer M, Costa C, Gimenez Arnau A, et al. Antihistamine-resistant chronic spontaneous urticaria remains undertreated: 2-year data from the AWARE study. Clin Exp Allergy. 2020;50(10):1166-1175. doi:10.1111/cea.13716
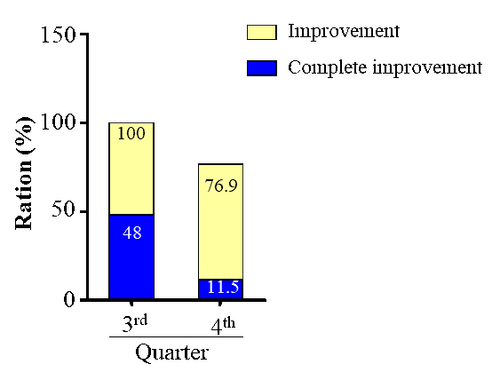
The Quality of life survey showed that after 4 weeks of treatment, averagely, 64.45% of the patients reported an improvement, while approximately 29.75% of those had a complete improvement. This was consistent with the result of UCT score reassessment.
V. CONCLUSION
Vinmec Healthcare system’s urticaria management program, in accordance with the reputable standards of GA2LEN has enhanced the quality of management and care for urticaria patients, especially chronic urticaria. The improvement will be shown by the following outcomes and some, as noted by an asterisk, have already been achieved:
- Improving the urticaria control test (UCT) score after 4 weeks of treatment.*
- Improving chronic urticaria – quality of life (CU-Q2oL) score after 4 weeks of treatment.*
- Screening for comorbidities with chronic urticaria such as autoimmune disease, allergic diseases, etc*
And these means that:
- Reducing the rate of complications associated with side effects of urticaria treatment, such as corticosteroids, herbal medicine of unknown origin, etc.
- Reducing opportunity costs caused by the burden of urticaria such as: reduce working hours; examination costs, ...
Moreover, we aim for a minimum of 70% of chronic urticaria patients to achieve good disease control score at the 1 year mark. We do believe that all of the efforts and treatment outcomes can lay a solid foundation to help Vinmec Times City become the first UCARE-certified center in Vietnam.
REFERENCES
- Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393-1414. doi:10.1111/all.13397
- Fricke J, Ávila G, Keller T, et al. Prevalence of chronic urticaria in children and adults across the globe: Systematic review with meta-analysis. Allergy. 2020;75(2):423-432. doi:10.1111/all.14037
- Maurer M, Abuzakouk M, Bérard F, et al. The burden of chronic spontaneous urticaria is substantial: Real-world evidence from ASSURE-CSU. Allergy. 2017;72(12):2005-2016. doi:10.1111/all.13209
- Thøgersen M. Home. UCARE. Accessed January 29, 2024. https://ga2len-ucare.com/
- Maurer M, Costa C, Gimenez Arnau A, et al. Antihistamine-resistant chronic spontaneous urticaria remains undertreated: 2-year data from the AWARE study. Clin Exp Allergy. 2020;50(10):1166-1175. doi:10.1111/cea.13716