This is an automatically translated article.
Posted by Master, Doctor Mai Vien Phuong - Gastrointestinal Endoscopy - Department of Medical Examination & Internal Medicine - Vinmec Central Park International HospitalEosinophils are one of the components of white blood cells produced in the bone marrow and are one of the cells that play a role in promoting inflammation, especially allergic inflammatory reactions. Therefore, large quantities of eosin can accumulate in tissues such as the esophagus, stomach, small intestine and sometimes in the blood when such individuals are exposed to the allergen.
Abbreviated terms in the article:
EoE: Eosinophilic esophagitis: esinophilic esophagitis GERD: Gastroesophageal reflux disease: Reflux esophagitis IL: Interleukin: Interleukin factor PPI: Proton pump inhibitor: Proton pump inhibitor Proton PPI-REE: Proton pump inhibitor-responsive esophagal: esophagitis If an increase in BCAT is found in the esophagus, 3 possibilities should be raised: VTQTCAT, GERD and PPI-responsive laryngeal BCAT (PPLREE).
Guidelines updated in 2011 described a new pathological phenotype, proton pump inhibitor-responsive esophageal eosinophilia (PPI-REE), referring to patients who appeared to have EoE clinically, but achieved complete remission after PPI treatment. Currently, PPI-REE must be formally excluded before EoE is diagnosed, as 30-40% of patients with suspected EoE are eventually diagnosed with PPI-REE. Interestingly, PPI-REE and EoE remain indistinguishable based on clinical, endoscopic and histological findings, esophageal pH monitoring, and measurement of tissue markers and cytokines associated with eosinophilic inflammation. acidosis, the acid-suppressing power of PPI therapy, is becoming obsolete. There is increasing evidence that PPI-REE is genetically and phenotypically indistinguishable from EoE and that PPI therapy alone can completely reverse allergic inflammation. As such, PPI-REE may constitute a subtype of EoE and PPI therapy may be the first step of treatment and diet/steroid may represent advanced therapy. It is possible that the term PPI-REE will soon be replaced by a PPI-responsive EoE. The mechanism why some patients respond to PPI therapy (PPI-REE) while others do not (EoE), remains to be elucidated.
1. Pathogenesis of PPI-REE
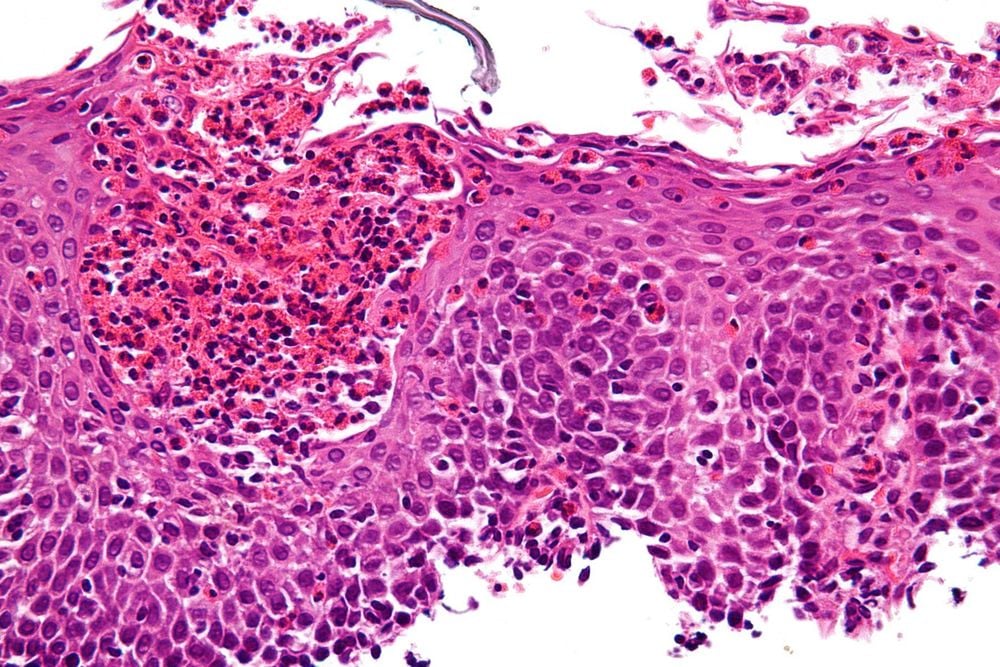
Since PPI-REE is clinically indistinguishable from EoE, and because esophageal acid exposure does not reliably explain PPI response, whether the pathogenesis of PPI-REE is similar? same as EoE? EoE is an immune-mediated condition in which, in genetically predisposed individuals, food or environmental antigens trigger a T-helper (Th2) type 2 mediated response despite cytokines such as interleukin (IL)-4, IL-5, and IL-13, which stimulate the esophagus to produce eotaxin-3 (an eosinophil chemotherapy agent). This is followed by recruitment and activation of eosinophils in the esophageal mucosa. Other mediators involved in the pathogenesis of EoE include transforming growth factor-β1, fibroblast growth factor, and thymic stromal lymphopoietin. Eotaxin-3 in the esophagus is a key promoter of eosinophil chemotaxis, activation and subsequent release of intracellular granules such as primary basic protein (MBP) and other inflammatory mediators. Prior investigations showed that MBP and eotaxin-3 were significantly up-regulated in the esophageal epithelium of EoE patients compared with GERD controls and could be used clinically to predict EoE status. Mast cell staining for tryptase has also been successful in differentiating EoE from GERD.
Recent studies have shown that these same markers are also increased in PPI-REE, and while there is a difference between EoE and GERD, there is no difference between EoE and PPI-REE. In a prospective study of 23 patients with PPI-REE, 50 patients with EoE, and 123 controls, immunohistochemical staining of esophageal biopsies for MBP, eotaxin-3, and mast cell tryptase revealed elevated levels of mast cell tryptase. all three markers in both EoE and PPI-REE. After a PPI trial, these markers returned to normal in PPI-REE, but remained elevated in EoE subjects. A similar prospective study by Molina-Infante et al comparing EoE and PPI-REE patients showed no significant difference in the gene expression levels of eotaxin-3 and IL-13 in whole plants. and of IL-5 in the distal esophagus. Expression levels of all three cytokines also decreased after PPI treatment in the PPI-REE group. Therefore, the available immunohistochemical data comparing EoE and PPI-REE suggest that similar inflammatory factors may be involved in their pathogenesis.
2. Genetic expression in PPI-REE
As EoE and PPI-REE are clinically and histologically indistinguishable, useful molecular biomarkers would be desirable. The EoE Diagnostic Panel (EDP) is a genetic test consisting of 94 genes differentially expressed in EoE and can reliably identify patients with EoE. When EDP was first evaluated in PPI-REE, the gene expression pattern of PPI-REE patients prior to PPI administration was very similar to that in EoE and included key EoE-related genes for chemotaxis. eosinophils (CCL26), mast cell genes (CPA3 and TPSAB2), inflammatory marker Th2, epithelial barrier gene, and tissue fibrosis marker. Furthermore, this genetic marker in PPI-REE was reversed after PPI treatment in a manner similar to that seen in EoE patients following diet or steroid treatment. In contrast, PPIs did not reverse molecular features in EoE.
Although the aforementioned study showed the majority of transcriptomes to overlap between PPI-REE patients and those with EoE, investigators found some significant differences between EoE and PPI-REE patients. (before their PPI testing) in basal expression of a panel of 10 genes. One gene, KCNJ2, surpassed the false discovery rate and was investigated further. KCNJ2 was expressed at high levels in the EoE group, but at low levels in normal controls and PPI-REE patients, and could predict PPI-REE versus EoE with 72% sensitivity and 72% specificity. KCNJ2 encodes the Kir2.1 potassium channel, which is abundant in the gastrointestinal mucosa and co-localizes with the H1-K1 ATPase/proton pump. The role of Kir2.1 in acid secretion may explain PPI response and additional studies are underway to evaluate the clinical utility of this marker. These new findings are provocative and support a potential shared immune-mediated pathogenesis between EoE and PPI-REE.
3. PPI reaction mechanism in PPI-REE
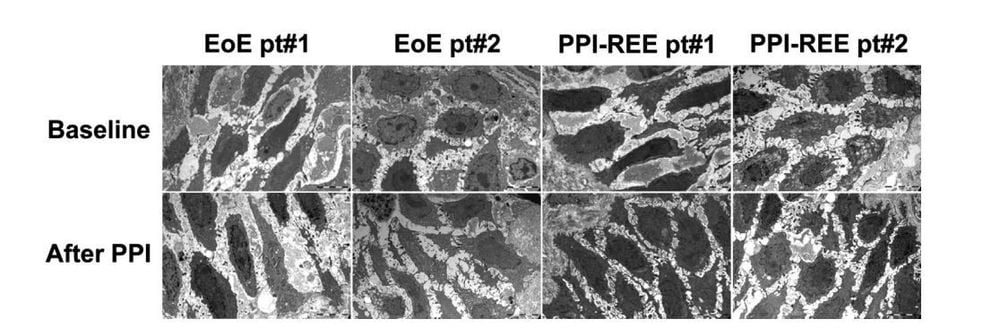
As there may be a common pathogenesis between EoE and PPI-REE, the mechanisms underlying PPI response in esophageal eosinophilia have also been investigated recently. Given the association between GERD and eosinophils, one hypothesis that the PPI response in PPI-REE is through a mechanism of acid reduction, the underlying treatment of GERD is not clinically clear. Even in the absence of symptoms, acid exposure from GERD can damage the esophageal epithelium, allowing entry of allergic antigens that induce an eosinophil response in the mucosa. A prospective study compared barrier integrity in 8 PPI-REE and 8 EoE patients, as measured by dilated intracellular space, electrical tissue impedance, transepithelial resistance, and flux molecule. The investigators found that, prior to PPI testing, barrier function was reduced in both PPI-REE and EoE, but recovered only in PPI-REE group after PPI test. However, more work is needed to elucidate the mechanisms of this improved barrier function.
Another new potential mechanism of PPI-REE is based on the anti-inflammatory effects of PPIs. Two recent studies demonstrated reduced Th2-related mediators in EoE cell lines treated with omeprazole, similar to that found in an asthma study in a canine lung model. Omeprazole blocked both IL-4- and IL-13-stimulated eotaxin-3 secretion from two EoE squamous cell lines in an experimental acid-free system. This is a strong demonstration of a proof of principle that PPIs may have an anti-inflammatory mechanism in esophageal eosinophilia. Furthermore, these studies suggest that the mechanism by which omeprazole suppresses cytokine-stimulated eotaxin-3 secretion is by preventing STAT6 from binding to the promoter region of the eotaxin-3 gene. These same investigators also described a distinct PPI effect by esophageal site in clinical biopsies, with a more prominent inhibition of eotaxin-3 in the proximal esophagus. Interestingly, unlike eotaxin-3 secretion by epithelial cells, eotaxin-3 secretion by fibroblasts is not blocked by PPIs, suggesting that there are additional processes that contribute to muscle pathogenesis of EoE and PPI-REE.
A third mechanism, which is just beginning to be investigated, addresses differential PPI metabolism as a potential mechanism of PPI response. An earlier case series describes four children with PPI-REE who lost PPI responsiveness (i.e. they developed recurrent symptoms and esophageal eosinophilia), despite being maintained on a steady dose of PPI. and no other confounding factors, and they ultimately met the diagnostic criteria for EoE. In a study presented in abstract by Molina-Infante et al., 72% of 46 long-followed PPI-REE patients had a sustained PPI response. Eight patients who relapsed with their PPI dose reduction subsequently responded to the PPI dose increase, and also had a rapid PPI-metabolizing phenotype on CYP450 enzyme testing. While all of these potential mechanisms of PPI response require further investigation, they point to a number of diverse and acid-independent ways by which PPIs can reduce esophageal eosinophils.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References
Swathi Eluri, MD 1, 2 and Evan S. Dellon, MD MPH. PPI-responsive esophageal eosinophilia and eosinophilic esophagitis: More similarities than differences. Curr Opin Gastroenterol. 2015 July; 31 (4): 309–315. Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. The Journal of allergy and clinical immunology. 2011;128(1):3–20. e6. quiz 1–2. Epub 2011/04/12. [PubMed] [Google Scholar] Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) The American journal of gastroenterology. 2013;108(5):679–692. quiz 93. Epub 2013/04/10. [PubMed] [Google Scholar]