This is an automatically translated article.
Posted by Dr. Truong Ngoc Hai - Resuscitation Doctor - Emergency Department - Vinmec Central Park International General Hospital
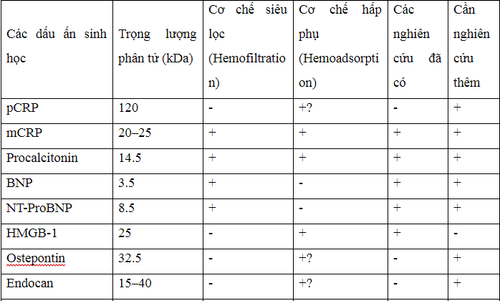
Today, with nearly 180 biomarkers have been identified, many biomarkers are being used by doctors to detect, monitor progress, evaluate prognosis of bacterial infections, sepsis and decided to stop taking antibiotics. However, these biomarkers may be eliminated during continuous dialysis, affecting the confidence in determining the severity of the infection. Until now, experts are still trying to find new biomarkers that are not eliminated by CRRT.
1. The most frequently used biomarkers in clinical practice
1.1. C-reactive Protein
C-reactive Protein (CRP) is mainly present as a monomer (mCRP) in the blood of patients with sepsis [5], which, due to its relatively small molecular weight (22-25 kDa) should be eliminated in all cases. all CRRT methods [4]. In addition, a significant amount of mCRP was also adsorbed on the membrane [2, 6]. Currently, many ICUs use filters with high adsorption capacity to increase mCRP clearance. Plasma concentrations of C-reactive Protein can be artificially low during CRRT, making CRP levels unreliable in the diagnosis or assessment of infection during CRRT [7].
CRP xuất hiện trong máu của bệnh nhân nhiễm khuẩn huyết
1.2. Procalcitonin (PCT)
In sepsis patients undergoing continuous venous hemodialysis (CVVH), PCT was detected in the ultrafiltration fluid of all patients [10]. Most PCT is eliminated via convection, but the adsorption mechanism also contributes to PCT clearance during the first hours of therapy [10]. The use of filters with high adsorption capacity further increased the clearance of CRP and PCT [6, 11].
1.3. Brain Natriuretic Factors
Biomarkers BNP and NT-proBNP have been applied recently in sepsis. Both biomarkers BNP and NT-proBNP are eliminated very readily by high-flux and low-flux membranes in CRRT due to their low molecular size [13], [14].
2. Cytokine/Chemokine biomarkers in sepsis
2.1. High Mobility Group 1 Protein (HMGB-1)
Although HMGB-1 has a relatively low molecular weight, HMGB-1 could theoretically be eliminated by convection filtration. However, in fact, HMGB-1 is only effectively eliminated via adsorption mechanism by filters with high adsorption capacity, especially acrylonitrile 69 (AN69-ST) surface-treated membranes [15] ].
Trong cơ chế lọc dối lưu, HMGB1bị thải trừ nhưng không hiệu quả
2.2. Osteopontin
Osteopontin is a negatively charged protein, with a molecular weight of about 32 kDa. There is no convincing evidence that continuous hemodialysis can clear osteopontin from the circulation [16]. However, CRRT uses new types of membranes, such as the AN69-ST membrane – due to its surface covered with a polyethylene biopolymer layer, it has a high positive charge density, in addition to its biocompatibility and high permeability, the membrane It also has a strong adsorption capacity, so it can increase the excretion of Osteopontin [6].
3. Biomarkers in sepsis associated with vascular endothelial damage – Endocan
Endocan is a novel endothelial-derived soluble dermatan sulfate proteoglycan with a molecular mass of about 15-40 kDa [17, 18]. Modern CRRT membranes can remove substances with molecular weights up to 35 kDa. When using a new filter with high adsorption capacity, the elimination of endocan through CRRT is increased [6]. Therefore, the confidence level of endocans in CRRT may be altered.
Endocan có thể bị thải trừ qua màng lọc CRRT
4. Biomarker in vasodilatation-associated sepsis – Proadrenomedullin (MR-proADM)
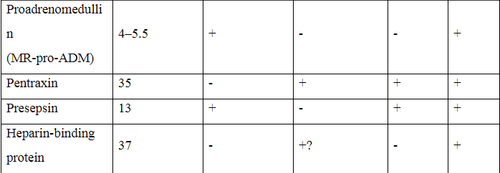
The molecular weight of MR-proADM is between 4 and 5.5 kDa, so it can also be removed by CRRT. The study by Mueller et al showed a significant reduction of MR-proADM (45.65%) if high-flux membranes were used [20] (especially with the 35 kDa filtration threshold of existing CRRT membranes). great [6]). More studies are needed to determine whether or not MR-proADM is eliminated by convection in CRRT.
5. Other biomarkers in the acute phase – Pentraxin
Pentraxin-3 (PTX3) is a glycoprotein released by endothelial and inflammatory cells upon activation of cytokines and endotoxins. PTX3 better reflects inflammation of local blood vessels and bacterial load than CRP [23]. Therefore, PTX3 may be a more appropriate marker than CRP in terms of severity and prognosis of necrotizing soft tissue infection. As reported by Hansen, there was a statistically significant association between high PTX3 levels and the occurrence of septic shock, amputation, the need for dialysis, and the risk of death in patients with tissue necrosis infection. soft [23].
In most patients, PTX3 in plasma is monomeric with a molecular weight of about 35 kDa [23] and can therefore theoretically be eliminated by CRRT. Recently, Schilder et al. demonstrated that although PTX3 is slightly adsorbed, the CVVH mode does not eliminate PTX3 by convection, resulting in unchanged plasma PTX3 concentrations [24]. It is necessary to further study the possibility of increasing the elimination of PTX3 with the CVVH method using membranes with high adsorption properties [24, 25].
Pentraxin-3 (PTX3) có mối liên quan đến tình trạng sốc nhiễm khuẩn
6. Biomarkers of cell markers in sepsis – Presepsin
Recently, sCD14-ST, also known as presepsin, has been identified as a potential biomarker of sepsis [26]. Presepsin is fragmented from a larger glycoprotein and has a molecular weight of about 13 kDa. The clearance of presepsin may be even higher than expected because the molecule can "stick" to a highly adsorbent filter [6].
7. Biomarkers of blood coagulation in sepsis - Heparin Binding Protein
Heparin Binding Protein (HBP), also known as a positively charged antimicrobial protein, has a molecular weight of 37 kDa [28] and can therefore be eliminated by CRRT via a convection mechanism [6]. Using membranes with high adsorption capacity can enhance HBP removal through adsorption [6].
The following table summarizes all biomarkers described in this review with information on molecular weight, clearance by convection and/or adsorption, noting the available research. and need more research. Thereby showing that all biomarkers can be eliminated via CRRT, especially when using a filter with high adsorption capacity. Therefore, there are no biomarkers that accurately reflect the infection status in patients undergoing CRRT. More studies are needed to evaluate and find new biomarkers that reliably reflect the infection status during CRRT. This task becomes even more complicated when the filter with high adsorption capacity has raised the filtration threshold up to 65 kDa.
Dấu ấn sinh học về đông máu trong nhiễm khuẩn huyết
References
1.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. 2. Honore PM, Jacobs R, De Waele E, Van Gorp V, Spapen HD. Biomarkers of inflammation during continuous renal replacement therapy: sensors, players, or targets? Blood Purif. 2014;38:102–3. 3. Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–71. 4. McFadyen JD, Kiefer J, Braig D, et al. Dissociation of C-reactive protein localizes and amplifes inflammation: evidence for a direct biological role of C-reactive protein and its conformational changes. Front Immunol. 2018;9:1351. 5. Taylor KE, van den Berg CW. Structural and functional comparison of native pentameric, denatured monomeric and biotinylated C-reactive protein. Immunology. 2007;120:404–11. 6. Dahaba AA, Elawady GA, Rehak PH, List WF. Procalcitonin and proinflammatory cytokine clearance during continuous venovenous haemofltration in septic patients. Anaesth Intensive Care. 2002;30:269–74. 7. Matsui T, Nakagawa T, Kikuchi H, Horio H, Hashimura K. The effect of continuous renal replacement therapy with the AN69ST membrane on inflammatory markers and the level of consciousness of hemodialysis patients with stroke: comparison with hemodialysis with low blood flow rate . Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2018;39:29–35. 8. Taylor R, Jones A, Kelly S, et al. A review of the value of procalcitonin as a marker of infection. Cureus. 2017;9:e1148. 9. Vijayan AL, Ravindran S, Saikant R, Lakshmi S, Kartik R. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51. 10. Level C, Chauveau P, Guisset O, et al. Mass transfer, clearance and plasma concentration of procalcitonin during continuous veno-venous hemofltration in patients with septic shock and acute oligoric renal failure. Crit Care. 2003;6:R160–6. 11. Honore PM, Jacobs R, Joannes-Boyau O, et al. Newly designed CRRT membranes for sepsis and SIRS—a pragmatic approach for bedside intensivists summarizing the more recent advances: a systematic structured review. ASAIO J. 2013;59:99–106. 12. N L, Zhang Y, Fan S, Xing J, Liu H. BNP and NT-proBNP levels in patients with sepsis. Front Biosci (Landmark Ed). 2013;18:1237–43. 13. Pirracchio R, Salem R, Mebazaa A. Use of B natriuretic peptide in critically ill patients. Biomark Med. 2009;3:541–7. 14. Wahl HG, Graf S, Renz H, Fassbinder W. Elimination of the cardiac natriuretic peptides B-type natriuretic peptide (BNP) and N-terminal proBNP by hemodialysis. Clin Chem. 2004;50:1071–4. 15. Yumoto M, Nishida O, Moriyama K, et al. In vitro evaluation of high mobility group box 1 protein removal witvarious membranes for continuous hemofltration. Ther Apher Dial. 2011;15:385–93. 16. Honore PM, Jacobs R, Hendrickx I, De Waele E, Van Gorp V, Spapen HD. To counteract or to clear high-mobility group box-1 protein in influenza A (H1N1) infection? That may become the question. Crit Care. 2015;19:401. 17. Honore PM, De Bels D, Attou R, Redant S, Gallerani A, Kashani K. Endocan removal during continuous renal replacement therapy: does it affect the reliability of this biomarker? Crit Care.2019;23:184. 18. Hureau M, Gaudet A, De Freitas Caires N, et al. Endocan is a reliable biomarker during continuous renal replacement therapy. Crit Care. 2019;23:296. 19. De Freitas Caires N, Gaudet A, Portier L, Tsicopoulos A, Mathieu D, Lassalle P. Endocan, sepsis, pneumonia, and acute respiratory distress syndrome. Crit Care. 2018;22:280. 20. Mueller T, Gegenhuber A, Kronabethleitner G, Leitner I, Haltmayer M, Dieplinger B. Plasma concentrations of novel cardiac biomarkers before and after hemodialysis session. Clin Biochem. 2015;48:1163–6. 21. Elke G, Bloos F, Wilson DC, et al. The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis—a secondary analysis of a large randomised controlled trial. Crit Care. 2018;22:79. 22. Honore PM, De Bels D, Attou R, Redant S, Kashani K. The challenge of removal of sepsis markers by continuous hemofltration. Crit Care. 2019;23:173. 23. Hansen MB, Rasmussen LS, Garred P, Bidstrup D, Madsen MB, Hyldegaard O. Pentraxin-3 as a marker of disease severity and risk of death in patients with necrotizing soft tissue infections: a, prospective, observational study. Crit Care. 2016;20:40. 24. Schilder L, Nurmohamed SA, ter Wee PM, et al. Putative novel mediators of acute kidney injury in critically ill patients: handling by continuous venovenous hemofltration and effect of anticoagulation modalities. BMC Nephrol. 2015;16:178. 25. Honore PM, Spapen HD. Pentraxin-3 to better delineate necrotizing soft tissue infection: not really! Crit Care. 2016;20:173. 26. Zhang X, Liu D, Liu YN, Wang R, Xie LX. The accuracy of presepsin (sCD14-ST) for the diagnosis of sepsis in adults: a meta-analysis. Crit Care. 2015;19:323. 27. Honore PM, Jacobs R, Hendrickx I, De Waele E, Van Gorp V, Spapen HD. Presepsin and sepsis-induced acute kidney injury treated with continuous renal replacement therapy: will another promising biomarker bite the dust? Crit Care. 2015;19:428. 28. Honore PM, De Bels D, Barreto Gutierrez L, Redant S, Spapen HD. Heparin-binding protein in sepsis: player! predictor! positioning? Ann Intensive Care. 2019;9:71. 29. Tverring J, Vaara ST, Fisher J, Poukkanen M, Pettilä V, Linder A, FINNAKI Study Group. Heparin-binding protein (HBP) prediction prediction of sepsis-related acute kidney injury. Ann Intensive Care. 2017;7:105. 30. Honore PM, Jacobs R, Hendrickx I, De Waele E, Van Gorp V, Spapen HD. ‘Biomarking’ infection during continuous renal replacement therapy: still relevant? Crit Care. 2015;19:232. 31. Schadler D, Pausch C, Heise D, et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: a randomized controlled trial. PLoS One. 2017;12:e0187015. 32. Honore PM, Hoste E, Molnár Z, et al. Cytokine removal in human septic shock: where are we and where are we going? Ann Intensive Care. 2019;9:56