This is an automatically translated article.
Article by Dr. Dr. Truong Ngoc Hai - Resuscitation Doctor - Emergency Resuscitation Department - Vinmec Central Park International General HospitalPlasma exchange (TPE) is a method of separating blood for the purpose of removing large molecules such as autoimmune antibodies, immune complexes, immunoglobulins, endotoxins or lipoproteins... Eliminate these substances in order to improve the clinical manifestations of the disease. In addition, TPE also helps to activate lymphocytes to control cytotoxicity, supplement beneficial proteins such as albumin, coagulation factors, to improve coagulation disorders [34].
Around the world, a number of studies in the intensive care units recorded the complication rate ranging from 11.1 to 45.5% [12],[42],[69] with the most complications of plasma exchange. mild and not requiring intervention such as allergies, hypocalcaemia, hypotension... Some serious complications such as transfusion-associated acute lung injury (TRALI), anaphylaxis were also noted. The rate of complications depends on many factors such as method of separation, fluid replacement, volume exchanged, vascular access, anticoagulation, and underlying disease [49].
1. Introduction
1.1 Development history Right from the Renaissance, the idea that the cause of disease was caused by toxins formed in the blood appeared and gradually led to measures such as using leeches, extracting blood for treatment. However, such measures caused many adverse effects due to blood loss and Louis XIII, George Washington may have died by this method [66]. Selective plasma removal along with the term “plasmapheresis” was first introduced and described in 1914 by John J. Abel, Rowntree and Turner of the Johns Hopkins University Pharmaceutical Laboratory. When conducting artificial kidney studies, they found that normal horses that would die on re-extraction could survive if the red blood cells were separated manually by centrifugation from the plasma and retransfused with the solution. Locke. However, it is not well received in clinical practice due to its complexity and time consuming [75]. The 1950s saw manual plasma separation become more widely and effectively used in the treatment of hyperviscosity syndrome in Waldenstrom's disease. In 1960, the study by Schawb and Fahey showed the undeniable benefit of plasma exchange and made it the standard treatment in Waldenstrom patients with hyperviscosity syndrome [64]. In 1959, plasma exchange was used for the first time in the treatment of immune-related diseases when Michael Rubinstein saved the life of a boy with Thrombotic Thrombotic Purpura (TTP) at the Hospital. Cedars of Lebanon, Los Angeles [75].
1965, marked the first appearance of the commercial line of continuous cell division IBM 2990. This invention was the result of a chance meeting between Emil J Freireich of the National Cancer Institute and the engineering team. Professor George Judson of IBM, whose son has been diagnosed with leukemia, is being treated here [21]. The introduction of this device was initially aimed at collecting granulocytes and platelets from donors to assist in the treatment of immunocompromised cancer patients. However, it at the same time solved important technical obstacles in plasma exchange making it easy for clinical application.
In 1974, DJ Wallace, based on the observations of Mart Mannik, proposed the therapeutic role of plasma exchange in systemic lupus erythematosus (SLE) and successfully applied it to a female SLE patient for the first time. resistant to corticosteroids and cytotoxic drugs [75]. In 1976, Lockwood's study published in the Lancet showed that plasma exchange plus immunosuppressants improved survival in patients with Goodpasture syndrome contributed to the widespread use of plasma exchange, not only in this syndrome but also in myasthenia gravis and TTP [44].
In 1978, plasma membrane replacement was introduced using the same principle as in dialysis in the hope of simplifying the procedure and not causing loss of other blood components. However, it is only in the last few years that this method has become really popular when advances in membrane fabrication have allowed them to be deployed on continuous dialysis machines. This saves the cost of purchasing machinery, as well as training the medical team when most nephrologists and critical care professionals are very familiar with the management of extracorporeal exchange systems [23]. .
In 1982, ASFA was established to bring together not only doctors, scientists but also nurses and staff directly operating blood separation equipment to build a forum for knowledge sharing, connection and promotion. promote learning and research in the field of hemodialysis. In 2007, ASFA published its fourth practice guide which classified the indications for hemodialysis based on evidence-based medicine for the first time. To date, eight publications have been published, with the latest edition in 2019 addressing plasma exchange in 84 conditions with 157 recommendations classified by GRADE [52].
1.2 Some Terms The term “apheresis” roughly translates to blood cleavage comes from the Greek “apairesos” or Roman “aphaeresis,” which means to divide, to separate by force, or to remove. . Hemodialysis describes several methods of separating blood into its components for removal or correction and then returned to the body.
Among the hemodialysis techniques, plasma exchange is the most common technique. By definition, “therapeutic plasma exchange” or “plasma exchange” is therapy that removes large amounts of a patient's plasma and requires the addition of a suitable replacement fluid such as fresh serum or albumin. “Plasmapheresis” removes a small amount of plasma from <15% of the patient's blood volume, usually without the need for fluid replacement, used in the production of albumin, IVIG, coagulation factors. However, the two terms are often used interchangeably, so they are not clearly distinguished in foreign literature.
2. Implementation method
2.1 Mechanism of action Blood volume accounts for about 7% of body weight, and is composed of 60% plasma and 40% blood cells. This percentage varies from person to person depending on gender, age, and a number of other factors. Plasma and interstitial fluid form extracellular fluid, but because of selective capillary permeability and the Donnan effect, plasma has a higher protein and positive ion ratio than interstitial fluid (about 2%). Practically their electrolyte composition can be considered to be similar. Besides electrolytes, plasma also contains glucose, amino acids, lipids, albumin, globulins, fibrinogen, clotting factors (Figure 1.1).[24]
![Hình ảnh: Các thành phần điện giải và không điện giải trong huyết tương “Nguồn: Guyton, 2016”[24]](/static/uploads/large_20220408_041611_952773_thay_huyet_tuong_1_max_1800x1800_png_de8357bc6e.png)
Hình ảnh: Các thành phần điện giải và không điện giải trong huyết tương “Nguồn: Guyton, 2016”[24]
Through plasma removal and replacement, TPE works to prevent or reduce target organ damage in diseases initiated and/or aggravated by circulating plasma pathogens such as drugs, plasma-bound toxins, immune complexes, cytokines, abnormal proteins, lipoproteins, etc. This elimination and regulation mechanism is considered to be the main mechanism for the therapeutic effect of TPE. In addition, TPE also helps to replenish a large amount of deficient plasma factors if fresh plasma substitutes are used, such as von Willebrand factor or complement in thrombotic microangiopathy. However, this mechanism does not explain the response times seen in some diseases. Some new evidence suggests that TPE, in addition to removing immunoglobulins, may modulate immunity through altering the number and activation of B and T lymphocytes, and altering the ratio of T-helper 1/T cells. 2, inhibit the production of interleukin-2 and interferon-ɤ, proliferation, and increase the susceptibility of lymphocytes to immunosuppressants or chemotherapy [59].
2.2 Plasma Separation Methods Initially, TPE was performed manually by drawing blood in repeated cycles, centrifuging to separate and discard the plasma, the remainder of the blood being returned to the body after add alternative translations. Today, TPE is performed automatically by two main methods: centrifugation and plasma membrane separation. TPE by centrifugation is popular in the US, in which the plasma membrane TPE method is popular in Germany and Japan [73].
2.2.1 Centrifugal TPE by centrifugation (cTPE) separates blood by placing the patient's blood continuously in a centrifuge chamber, usually using citrate as an anticoagulant. Centrifugal chamber separates blood based on specific gravity of blood and plasma cell components. The portion of blood cells is then added to the replacement solution, which can be serum or albumin, and returned to the patient.
Centrifugal chamber rotates at a high speed of about 2000-2500 rpm, maximum is 6000 rpm, creating centrifugal force to separate components of blood according to gravity from the center to the periphery: plasma, platelets, white blood cells, red blood cells. The removal of plasma through the sieve at the center of the spindle is also aided by a "buffy coat" made up of platelets, monocytes, and lymphocytes that form a membrane separating erythrocytes. The effectiveness of plasma exchange depends on the size, speed of the centrifuge generating machine, and the time the blood is in the centrifuge system, also known as the “dwell time” [76].
![Hình ảnh: Kỹ thuật TPE bằng phương pháp quay ly tâm “Nguồn: Williams, 2014” [76]](/static/uploads/large_20220408_041625_430446_thay_huyet_tuong_2_max_1800x1800_png_2165941f45.png)
Hình ảnh: Kỹ thuật TPE bằng phương pháp quay ly tâm “Nguồn: Williams, 2014” [76]
The intermittent method requires only one venous collection site, and this is the same route used to return blood. This method requires more time and volume of priming, so it is not suitable for low-birth-weight patients such as children. The continuous method requires two venipuncture sites for simultaneous collection and return of blood. Large amounts of blood can be treated at the same time with small volumes of primers. This method is not suitable for patients who have difficulty establishing the line, as well as requiring restriction of movement that may cause discomfort to the patient. 2.1.2 Membrane plasma separation TPE by membrane plasma separator (mTPE) uses the same principle as continuous ultrafiltration mode hemodialysis. The blood is passed through a highly permeable membrane with a filter hole that allows plasma proteins to pass through but retains the blood cells. The pore size is usually ≤ 0.6 μm, so it is easy to retain the smallest blood cells, platelets (3 μm). The plasma exchange efficiency of the membrane depends on the filtration rate and on the properties of the membrane. To avoid membrane coagulation during plasma exchange, the filtration fraction should be about 30-35% of plasma, especially when the patient is overweight. Therefore, for plasma purification, the replacement volume needs to be about 3-4 times the blood volume. The plasma membrane is not selective, therefore, in addition to pathogens, it may remove beneficial factors from the plasma [76].
![Hình ảnh: Kỹ thuật TPE bằng màng tách huyết tương “Nguồn: Williams, 2014”[76]](/static/uploads/large_20220408_041704_477889_thay_huyet_tuong_3_max_1800x1800_png_2e44f7cc61.png)
Hình ảnh: Kỹ thuật TPE bằng màng tách huyết tương “Nguồn: Williams, 2014”[76]
Intrinsic membrane factors include pore size, plasma distribution. The factor affecting filtration efficiency mainly depends on the molecular size of the substance, plasma exchange will be effective in substances ranging in size from albumin (67,000D) to B lipoprotein (2,400,000D). The efficiency of filtration may also depend on other factors such as: deposition factors, albumin adsorption, deposition of substances on the membrane that will reduce the filtration efficiency of the membrane [51].
2.3 Differences between the two plasma exchange methods [74] Comparison of the two TPE methods
| Đặc điểm | Quay ly tâm | Màng tách huyết tương |
| Cơ chế | Lực ly tâm | Lọc qua màng lọc |
| Tốc độ máu (ml/ phút) | 10-150 | 40-150 |
| Hiệu quả loại bỏ huyết tương (%) | 80-90 | 30 |
| Tốc độ loại bỏ huyết tương (ml/phút) | Thay đổi | 30 |
| Kháng đông | Citrate | Heparin |
| Thể tích máu trong vòng thay thế | 180 | 125 |
| Kích thước phân tử giới hạn | Không giới hạn | 3 triệu Daltons |
| Dịch thay thế | Albumin, plasma | Albumin, plasma |
“Source: Williams, 2014”[76]
Few studies directly compare priming and preparation times of TPE methods. Basically, cTPE takes less time because there is no need to calibrate the equalization system like mTPE. Spectra Optica system needs about 11 minutes to prepare while mTPE systems like Diapact (B.Braun Avitum, Germany) need about 20-24 minutes, Prisma system (Baxter, USA) using TPE 2000 membrane kit is 40 minutes [74].
Plasma removal efficiency reflects the ratio of plasma removed to the amount of plasma processed. A study that directly compared the Spectra Optica system and the two mTPE systems showed that the Spectra Optica had a plasma removal efficiency of approximately 80-90% significantly higher than that of the Diapact system of 53% and the OctoNova with membranes. Plasmaflo OP-05W is 27%. The main reason for this difference is that the processing time of mTPE is longer than that of cTPE with the same plasma volume [26],[35].
The minimum blood draw rate of mTPE is 40ml/min, depending on the membrane, to create the required transmembrane pressure in blood separation, ensuring an acceptable processing time. Therefore, mTPE often requires a central venous line, which limits access as well as increases the risk of sepsis compared with cTPE requiring a peripheral vein only. In addition, because of the low plasma elimination efficiency, most anticoagulation will be returned to the body when using mTPE, causing the risk of cirtrate toxicity and hypocalcemia if citrate anticoagulant is used, so anticoagulation used in mTPE usually heparin. The cleavage time was also significantly shorter in cTPE, resulting in plasma exchange times required in cTPE about two-thirds that of mTPE when the same substitution dose was performed [74].
Volume of replacement:
Plasma is replaced through plasma removal and the remaining plasma is diluted with the replacement fluid, so solutes cannot be completely removed from the blood. Given the hypothesis that the intravascular compartment is an independent compartment when the plasma exchange time is relatively short, the effect of TPE is reflected in the remaining intravascular pathogen, which is dependent on the volume of plasma transfected, independent of method of splitting and following the rules:
y: is the concentration of solute after plasma exchange
y0: is the concentration of solute before plasma exchange
x: is the number of times the patient's plasma volume is changed
This equation was first described by Kaplan when studying the effectiveness of TPE in removing IgG in 18 patients with 102 times TPE using a simple plasma volume estimation formula: (0.065 x Weight) x (1-Hct) [34]. It has been shown that TPE with a substitution of 1-1.5 times the plasma volume will remove about 60-70% of the initial solute concentration. In addition, the volume of distribution and the half-life (T1/2) of the solute play an important role in elimination. For example, IgM is distributed mainly (78%) intravascularly with a T1/2 of 5 days, so it can be effectively removed with only a few TPE sessions. However, if IgG is ≤ 45% of intravascular volume with a T1/2 of 21 days, TPE needs to be repeated at reasonable intervals for effective removal [34]. A period of 24–48 hours will allow the pathogen to regenerate and reach equilibrium between the intravascular and extravascular compartments.
TPE with substitution volume >1.5 times plasma volume removes very few large molecules and increases substitution time and cost. However, in cases of acute liver failure where the primary goal is not to remove antibodies, high-volume TPE combined with high-dose continuous hemodialysis can help remove toxins of a very diverse molecular weight. produced in liver failure. In addition, high-volume TPE with FFP replacement fluid helps to regulate coagulation without causing fluid overload like conventional FFP infusion [7].
It is clear that the “one size fit all” strategy cannot be applied in plasma exchange dose selection for different diseases. Based on the knowledge of the pathogen, the severity, the type of fluid replacement, and the adverse effects of TPE, it is necessary for the treating physician to select the appropriate volume, frequency and duration of replacement ( Figure 1.5) [25].
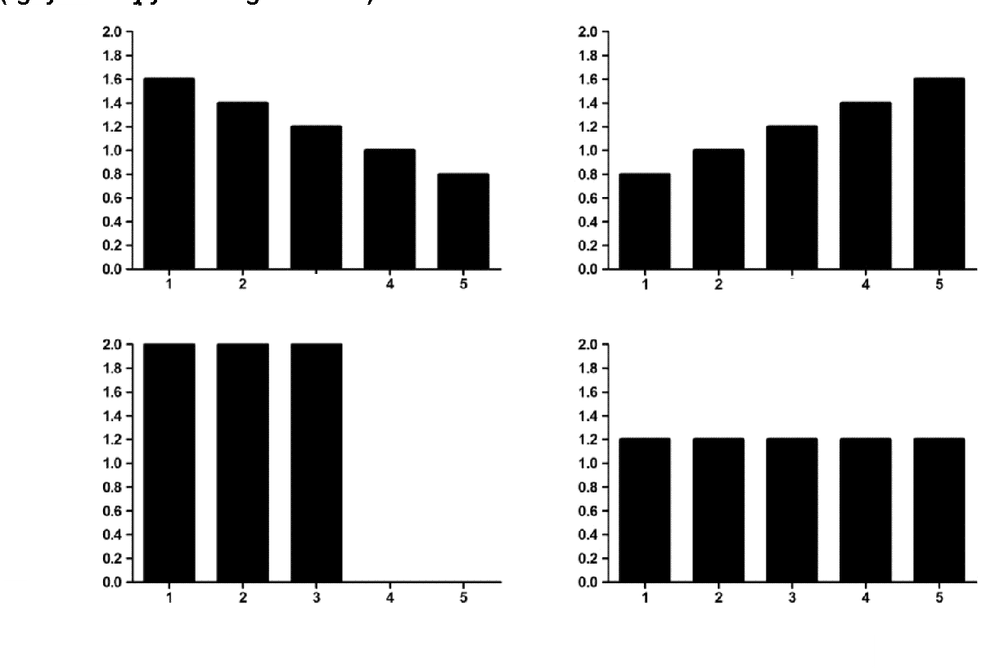
Hình ảnh: Một số gợi ý phân phối thể tích huyết tương theo thời gian
The total volume of replacement is 6 times the calculated plasma volume distributed over time in four different ways: A. descending (TTP), B. ascending (desensitized), C. high volume (sepsis, liver failure) and D. equal doses (Guillain-Barre syndrome).
“Source: Hafer, 2017”[25]
Anticoagulation:
Like all extracorporeal support modalities, TPE, whether using membranes or centrifugation, requires anticoagulation to ensure proper functioning. system. Choosing the type of anticoagulation, the dose needs to consider many factors such as blood clotting status, pathology, TPE modality as well as understanding how to monitor, detect and handle complications to ensure safety during the procedure. onion.
For cTPE, citrate anticoagulation is used, which acts by binding to ionized calcium preventing the initiation of the coagulation cascade that occurs in the TPE system. Most of the ionized calcium concentration will return to normal levels when the blood is returned to the body because ionized calcium stores together with citrate metabolism in the liver should significantly affect the patient's blood coagulation. However, the most common complication is hypocalcaemia with symptoms ranging from paresthesia to QT prolongation. With the goal of anticoagulation and minimizing systemic complications due to hypocalcaemia, the citrate dose should be limited to 1.0 - 1.8 mg/kg/min, approximated by maintaining a certain fluid ratio. citrate/whole blood and blood rate based on patient weight. In addition, it is important to note the patient's citrate metabolism (liver failure, shock), the amount of citrate in each type of replacement fluid (FFP contains up to 14% citrate by volume). Prophylaxis of hypocalcaemia is by infusion of 10 mL of 10% calcium gluconate over 15 minutes during the half cycle. Other authors have suggested that citrate toxicity can be well controlled with oral calcium tablets and that intravenous calcium should only be used in symptomatic patients [74].
For mTPE, heparin is the commonly used anticoagulant, with the advantage of being relatively inexpensive and having a short half-life (20 minutes to 2.5 hours). Unlike citrate, the anticoagulant effect of heparin is not limited to the TPE system but also affects the coagulation of the patient increasing the risk of bleeding. At TPE initiation, some typically bolus 1,000–3,000 UI, and deliver 1,000–2,000 UI/hour continuously, maintaining APTT(R) at 1.5–2.5 or APTT 50–60 seconds. For patients on warfarin therapy, this is the anticoagulation of choice. Heparin can cause heparin-induced thrombocytopenia (HIT), which should be suspected when thrombocytopenia with or without thrombosis, dialysate coagulation, and the use of an alternative anticoagulant (eg, argatroban). The heparin-PF4 antibody test is diagnostic. Besides, the antithrombotic effect of heparin is influenced by antithrombin, and heparin resistance may occur if the antithrombin is low, congenital or acquired. Note that heparin is an anion that will be adsorbed by cation membranes or anion exchange resins when bilirubin absorbent membranes are used [51].
Alternative fluid options:
The most commonly used substitute is 5% albumin in physiological saline. Unlike FFP, this solution has the main advantage of avoiding transfusion reactions (anaphylaxis, TRALI) and blood-borne diseases. The disadvantages are its high cost compared with FFP and possible coagulopathy, as well as non-selective removal of immunoglobulins. 5% albumin has a higher oncotic pressure than plasma (18 vs 22 torr), increasing intravascular volume thereby limiting hypovolemia. Albumin has a K+ concentration lower than 2mmol/L, so it is important to pay attention to compensate for 4 mmol per liter of albumin to avoid lowering K+ after infusion. Albumin is the most expensive part of the TPE process so the idea of using albumin at a lower concentration (3.5%) is adopted by some centers. However, they have been shown to increase the incidence of hypotension compared with albumin by 5% [49].
FFP is of limited use in certain conditions requiring supplementation of deficient plasma factors such as coagulation factors, ADAMTS13 when treating TTP, and to prevent dilution-induced coagulopathy in patients with bleeding disorders. massive blood. It should be noted that anaphylactoid reactions can be up to 20% including fever, chills, rash, wheezing, and hypotension. Diphenhydramine 50mg can be used before TPE. For patients with known sensitivity to FFP, 40 mg of solumedrod or 50 mg of prednisone administered 13 hours, 7 hours, and 1 hour before the combination of diphenhydramine 50 mg and 25 mg of epherine 1 hour before TPE was recommended [34]. If there is anaphylaxis, handle according to the anaphylactic shock protocol of the Ministry of Health.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
TÀI LIỆU THAM KHẢO
Tiếng Việt:
Hoàng Đức Chuyên (2012), Nghiên cứu đặc điểm lâm sàng và điều trị viêm tụy cấp tăng triglyceride, Luận văn Thạc sĩ Y học, Trường Đại Học Y Hà Nội. Nguyễn Minh Đức (2017), Nghiên cứu đặc điểm lâm sàng, chẩn đoán điện và kết quả điều trị hội chứng Guillain – barré bằng phương pháp thay huyết tương , Luận văn Tiến sĩ Y học, Viện Nghiên Cứu Khoa Học Y Dược Lâm Sàng 108. Huỳnh Thị Thu Hiền (2020), "Liệu pháp thay huyết tương ở bệnh nhân nặng", Tạp chí Y Học Thành Phố Hồ Chí Minh , 24 (2), tr. 202-206. Thông Võ Duy (2021), “Tăng trirelycerie máu rất nặng ở bệnh nhân viêm tụy cấp: yếu tố nguy cơ và kết cục lâm sàng”, Tạp chí Y học Việt Nam , 2, tr. 49-55. Lê Quang Thuận (2017), Nghiên cứu hiệu quả điều trị viêm gan nhiễm độc cấp nặng bằng biện pháp thay huyết tương tích cực , Luận văn Tiến sĩ Y học, Viện Nghiên Cứu Khoa Học Y Dược Lâm Sàng 108. Võ Thị Đoan Thục (2020), Nghiên cứu hiệu quả của thay huyết tương trong điều trị viêm tụy cấp nặng do tăng triglyceride máu tại Bệnh viện Chợ Rẫy , Luận văn Chuyên khoa 2, Đại Học Y Dược Huế. Tiếng anh:
7. Ahmed S., Kaplan A. (2020), "Therapeutic Plasma Exchange Using Membrane Plasma Separation", Clin J Am Soc Nephrol , 15 (9), pp. 1364-1370.
8. Apter A. J., Kaplan A. A. (1992), "An approach to immunologic reactions associated with plasma exchange", J Allergy Clin Immunol , 90 (1), pp. 119-24.
9. Basic-Jukic Nikolina, Kes Petar, Glavas-Boras Snjezana, et al. (2005), "Complications of Therapeutic Plasma Exchange: Experience With 4857 Treatments". Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, The Japanese Society for Dialysis Therapy , 9, pp. 391-395.
10. Beech C., Kumar D., Hendrickson J., et al. (2019), "Cryoglobulinemia as a Possible Primer for TRALI: Report of a Case". Lab Med, 50 (3), pp. 313-319 .
11. Bendapudi P. K., Hurwitz S., Fry A., Marques M. B., et al. (2017), "Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study", Lancet Haematol , 4 (4), pp. 157-164.
12. Calça Rita, Gaspar Ana, Santos Afonso, et al. (2020), "Therapeutic plasma exchange in patients in a portuguese ICU", Portuguese Journal of Nephrology & Hypertension , 34 (1), pp. 21-25.
13. Clebone A. (2019), "Transfusion reactions and cognitive aids". Curr Opin Anaesthesiol , 32 (2), pp. 242-246.
14. Colling M., Sun L., Upadhyay V., et al. (2020), "Deaths and complications associated with the management of acute immune thrombotic thrombocytopenic purpura", Transfusion , 60 (4), pp. 841-846.
15. Cordoba J. P., Larrarte C., Medina M. C. (2015), "Experience in therapeutic plasma exchange by membrane filtration at an academic center in Colombia: Registry of the first 500 sessions", J Clin Apher , 30 (6), pp. 347-52.
16. Daga Ruiz D., Fonseca San Miguel F., González de Molina F. J., et al. (2017), "Plasmapheresis and other extracorporeal filtration techniques in critical patients", Medicina Intensiva , 41 (3), pp. 174-187.
17. Erkurt M. A., Sarici A., Kuku I., et al. (2021), "The effect of therapeutic plasma exchange on management of HELLP Syndrome: The report of 47 patients", Transfus Apher Sci , 60 (5), pp. 1-3.
18. Evans L., Rhodes A., Alhazzani W., et al. (2021), "Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021 ", Intensive Care Med , 47 (11), pp. 1181-1247.
19. Fleischmann C., Scherag A., Adhikari N. K., et al. (2016), "Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations", Am J Respir Crit Care Med , 193 (3), pp. 259-72.
20. Fortenberry J. D., Nguyen T., Grunwell J. R, et al. (2019), "Therapeutic Plasma Exchange in Children With Thrombocytopenia-Associated Multiple Organ Failure: The Thrombocytopenia-Associated Multiple Organ Failure Network Prospective Experience", Crit Care Med , 47 (3), pp. 173-181.
21. Freireich E. J. (2011), "Leukocyte transfusion and the development of the continuous-flow blood cell separator", Transfus Med Rev , 25 (4), pp. 344-50.
22. Gajic O, Rana R., Winters J. L, et al. (2007), "Transfusion-related acute lung injury in the critically ill: prospective nested case-control study" . Am J Respir Crit Care Med, 176 (9), pp. 886-891.
23. Gashti C. N., Andreoli D. C., Patel D. (2018), "Membrane-based therapeutic plasma exchange (mTPE): Technical and clinical experience", J Clin Apher , 33 (1), pp. 38-45.
24. Guyton (2016), Guyton and Hall Textbook of Medical Physiology 13th , W B Saunders, Philadelphia, chapter 25, pp. 307-308 .
25. Hafer C., Kielstein J. T. (2017), "Pro: High dose of therapeutic plasma exchange-mind the gap!", Nephrol Dial Transplant , 32 (9), pp. 1457-1460.
26. Hafer C., Golla P., Gericke M., et al. (2016), "Membrane versus centrifuge-based therapeutic plasma exchange: a randomized prospective crossover study", Int Urol Nephrol , 48 (1), pp. 133-138 .
27. Hisamichi Mikako, Kawarazaki Hiroo, Oroku Masato, et al. (2016), "Risk factors for allergic reaction at initial therapeutic plasma exchange in a single-center study: beware of high rates of severe allergic reaction", Renal Replacement Therapy , 2 (1), pp. 2-5.
28. Horvatits T., Drolz A., Trauner M., et al. (2019), "Liver Injury and Failure in Critical Illness", Hepatology , 70 (6), pp. 2204-2215.
29. Hotchkiss Richard S., Moldawer Lyle L., Opal Steven M., Reinhart Konrad, Turnbull Isaiah R., et al. (2016), "Sepsis and septic shock", Nature Reviews Disease Primers , 2 (1), pp. 1-21.
30. Hu Lunyang, Wang Baoli, Jiang Yong, et al. (2021), "Risk factors for transfusion-related acute lung injury". Respiratory Care , pp. 08829.
31. Huang Y. K., Tan D. M., Xie Y. T., et al. (2012), "Randomized controlled study of plasma exchange combined with molecular adsorbent re-circulating system for the treatment of liver failure complicated with hepatic encephalopathy", Hepatogastroenterology , 59 (117), pp. 1323-1326.
32. Kandiah P. A., Olson J. C., Subramanian R. M. (2016), "Emerging strategies for the treatment of patients with acute hepatic failure", Curr Opin Crit Care , 22 (2), pp. 142-151.
33. Kaplan A. (2012), "Complications of apheresis", Semin Dial , 25 (2), pp. 152-158.
34. Kaplan A. A. (2013), "Therapeutic plasma exchange: a technical and operational review ", J Clin Apher , 28 (1), pp. 3-10.
35. Kes P., Janssens M. E., Bašić-Jukić N., Kljak M. (2016), "A randomized crossover study comparing membrane and centrifugal therapeutic plasma exchange procedures, Transfusion , 56 (12), pp. 3065-3072.
36. Khandelwal P., Thomas C. C., Rathi B. S., Hari P., Tiwari A. N., et al. (2019), "Membrane-filtration based plasma exchanges for atypical hemolytic uremic syndrome: Audit of efficacy and safety", J Clin Apher, 34 (5), pp. 555-562.
37. Kuldanek S. A., Kelher M., Silliman C. (2019), "Risk factors, management and prevention of transfusion-related acute lung injury: a comprehensive update", Expert Rev Hematol , 12 (9), pp. 773-785.
38. Kundrapu S., Datla S., Griffin V., Maitta R. W. (2019), "Adverse events during apheresis: A 10-year experience at a tertiary academic medical center", J Clin Apher , 34 (5), pp. 528-536.
39. Lahmer T., Messer M., Schnappauf C., Rasch S., Fekecs L., et al. (2017), "Impact of Therapeutic Plasma Exchange on Hemodynamic Parameters in Medical Intensive Care Unit Patients: An Observational Study", Artif Organs , 41 (2), pp. 204-209.
40. Larsen F. S., Schmidt L. E., Bernsmeier C., Rasmussen A., Isoniemi H., et al. (2016), "High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial" , J Hepatol , 64 (1), pp. 69-78.
41. Lee K. C., Stadlbauer V., Jalan R. (2016), "Extracorporeal liver support devices for listed patients", Liver Transpl , 22 (6), pp. 839-48.
42. Lemaire A., Parquet N., Galicier L., Boutboul D., Bertinchamp R., et al. (2017), "Plasma exchange in the intensive care unit: Technical aspects and complications", J Clin Apher , 32 (6), pp. 405-412.
43. Li Mao-Qin, Ti Jun-Xiang, Zhu Yun-Hang, et al. (2014), "Combined use of non-biological artificial liver treatments for patients with acute liver failure complicated by multiple organ dysfunction syndrome", World journal of emergency medicine , 5, pp. 214-217.
44. Lockwood C. M., Rees A. J., Pearson T. A., et al. (1976), "Immunosuppression and plasma-exchange in the treatment of Goodpasture's syndrome", Lancet , 1 (7962), pp. 711-5.
45. Mateen Farrah, Gastineau Dennis (2008), "Transfusion Related Acute Lung Injury (TRALI) after Plasma Exchange in Myasthenic Crisis". Neurocritical care , 8, pp. 280-282.
46. Meyer D. E., Reynolds J. W., Hobbs R., et al. (2018), "The Incidence of Transfusion-Related Acute Lung Injury at a Large, Urban Tertiary Medical Center: A Decade's Experience". Anesth Analg , 127 (2), pp. 444-449.
47. Miklaszewska M., Korohoda P., Zachwieja K., et al. (2017), "Filter Size Not the Anticoagulation Method is the Decisive Factor in Continuous Renal Replacement Therapy Circuit Survival", Kidney Blood Press Res , 42 (2), pp. 327-337.
48. Millar Jonathan E., Fanning Jonathon P., McDonald Charles I., et al. (2016), "The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology", Critical Care , 20 (1), pp. 387.
49. Mokrzycki M. H., Balogun R. A. (2011), "Therapeutic apheresis: a review of complications and recommendations for prevention and management", J Clin Apher , 26 (5), pp. 243-8.
50. Mortzell Henriksson M., Newman E., Witt V., et al. (2016), "Adverse events in apheresis: An update of the WAA registry data", Transfus Apher Sci , 54 (1), pp. 2-15.
51. Noiri Eisei (2014), “Complication”, The Concise Manual of Apheresis Therapy , Springer, Japan, chapter 17, pp. 177-224.
52. Padmanabhan A., Connelly-Smith L., Aqui N., Balogun R. A., Klingel R., et al. (2019), "Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue", J Clin Apher , 34 (3), pp. 171-354.
53. Palma-Garcia L., Velasquez-Rimachi V., Pezo-Pezo A., Roig J., Perez-Villegas J. (2018), "Therapeutic plasma exchange: Experience in a third level hospital, 2013-2016, Lima (Peru)" . J Clin Apher , 33 (4), pp. 480-485.
54. Paton E., Baldwin I. C. (2014), "Plasma exchange in the intensive care unit: a 10 year retrospective audit", Aust Crit Care , 27 (3), pp. 139-144.
55. Paugam-Burtz C., Levesque E., Louvet A., Thabut D., Amathieu R., et al. (2020), "Management of liver failure in general intensive care unit", Anaesth Crit Care Pain Med , 39 (1), pp. 143-161.
56. Puppe B., Kingdon E. J. (2014), "Membrane and centrifugal therapeutic plasma exchange: practical difficulties in anticoagulating the extracorporeal circuit", Clinical kidney journal , 7 (2), pp. 201-205.
57. Rajagopal K. (2019), "Left Ventricular Distension in Veno-arterial Extracorporeal Membrane Oxygenation: From Mechanics to Therapies", Asaio j , 65 (1), pp. 1-10.
58. Ranganathan Lakshmi, Menon Rema, Ramakrishnan Nagarajan, et al (2019), "Therapeutic Plasma Exchange Practices in Intensive Care Unit", Indian journal of critical care medicine , 23 (7), pp. 336-338.
59. Reeves H. M., Winters J. L. (2014), "The mechanisms of action of plasma exchange", Br J Haematol , 164 (3), pp. 342-51. .
60. Rimmer Emily, Houston Brett L., Kumar Anand, Abou-Setta Ahmed M., Friesen Carol, et al. (2014), "The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis", Critical care , 18 (6), pp. 699-699.
61. Roubinian N. (2018), "TACO and TRALI: biology, risk factors, and prevention strategies", Hematology Am Soc Hematol Educ Program , 2018 (1), pp. 585-594.
62. Sarode Ravi, Bandarenko Nick, Brecher Mark E., Kiss Joseph E., Marques Marisa B., et al. (2014), "Thrombotic thrombocytopenic purpura: 2012 American Society for Apheresis (ASFA) consensus conference on classification, diagnosis, management, and future research", Journal of Clinical Apheresis , 29 (3), pp. 148-167.
63. Schmidt J. J., Asper F., Einecke G., et al. (2018), "Therapeutic plasma exchange in a tertiary care center: 185 patients undergoing 912 treatments - a one-year retrospective analysis", BMC Nephrol , 19 (1), pp. 12.
64. Schwab P. J., Fahey J. L. (1960), "Treatment of Waldenstrom's macroglobulinemia by plasmapheresis", N Engl J Med , 263, pp. 574-9.
65. Sengul Samanci N., Ayer M., Gursu M., et al. (2014), "Patients treated with therapeutic plasma exchange: a single center experience"., Transfus Apher Sci , 51 (3), pp. 83-89.
66. Shumak Kenneth H., Rock Gail A. (1984), "Therapeutic Plasma Exchange", N Engl J Med, 310 (12), pp. 762-771.
67. Simetka O., Klat J., Gumulec J., Dolezalkova E, et al. (2015), "Early identification of women with HELLP syndrome who need plasma exchange after delivery", Transfus Apher Sci , 52 (1), pp. 54-59.
68. Stravitz R. Todd, Lee William M. (2019), "Acute liver failure", The Lancet , 394 (10201), pp. 869-881.
69. Szczeklik W., Wawrzycka K., Włudarczyk A., et al. (2013), "Complications in patients treated with plasmapheresis in the intensive care unit", Anaesthesiol Intensive Ther, 45 (1), pp. 7-13.
70. Toy P., Gajic O., Bacchetti P., et al. (2012), "Transfusion-related acute lung injury: incidence and risk factors", Blood , 119 (7), pp. 1757-67.
71. Tschope C., Ammirati E., Bozkurt B., Caforio A. L. P., Cooper L. T., et al. (2021), "Myocarditis and inflammatory cardiomyopathy: current evidence and future directions", Nat Rev Cardiol , 18 (3), pp. 169-193.
72. Vlaar A. P., Hofstra J. J., Determann R. M., et al. (2011), "The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: a prospective nested case-control study". Blood , 117 (16), pp. 4218-25.
73. Vlaar Alexander P. J., Toy Pearl, Fung Mark, et al. (2019), "A consensus redefinition of transfusion-related acute lung injury", Transfusion , 59 (7), pp. 2465-2476.
74. Wakelin Stuart, Janssens Michiel Etienne (2018), "Centrifugal and Membrane Therapeutic Plasma Exchange – A Mini-review", European Oncology & Haematology , 14 (2), pp. 105.
75. Wallace D. J. (1999), "Apheresis for lupus erythematosus", Lupus , 8 (3), pp. 174-180. .
76. Williams M. E., Balogun R. A. (2014), "Principles of separation: indications and therapeutic targets for plasma exchange" , Clin J Am Soc Nephrol , 9 (1), pp. 181-90.
77. Winters J. L. (2012), "Plasma exchange: concepts, mechanisms, and an overview of the American Society for Apheresis guidelines", Hematology Am Soc Hematol Educ Program , 2012 , pp. 7-12.
78. Yilmaz A. A., Can O. S., Oral M., et al. (2011), "Therapeutic plasma exchange in an intensive care unit (ICU): a 10-year, single-center experience", Transfus Apher Sci , 45 (2), pp. 161-166 .
79. Zhao Y., Garrity D., Graves M., Linden J., St Pierre P., et al. (2019), "Optimization of infusional calcium gluconate for prevention of hypocalcemic reactions during therapeutic plasma exchange" , J Clin Apher , 34 (6), pp. 656-660.