This is an automatically translated article.
Posted by Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International Hospital
There have been recent advances in the development and testing of AI and various machine learning (ML) algorithms to improve the ability to identify dysplastic and malignant mucosa.
The role of artificial intelligence in Barrett's esophagus (Part 1)
1.What is artificial intelligence AI?
Previously, computer algorithms were trained to classify a patient's likelihood of early esophageal cancer based on symptoms or to compare a patient's biopsied cDNA microchip with cancer samples known early stage esophagus.
These methods have brought us closer to the correct diagnosis of dysplasia and mucosal malignancy, but their sensitivity/specificity cannot match those outlined in the Preservation and Incorporation of the American Gastrointestinal Endoscopy Association (PIVI) Valuable Endoscopy for New Technologies. The PIVI criteria recommend that sensitivity be at least 0.90, specificity of at least 0.80, and negative predictive value of at least 0.98 for the detection of HGD or Barrett's esophagus. AI uses several methods of ML.
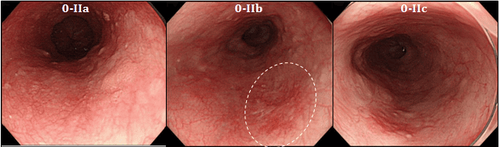
2.Cognitive Neural Networks (CNN) in AI
A commonly used method is cognitive neural networks (CNN). In CNN, each node (or "neuron") is connected to other nodes in a way that mimics a real human neural network. Several layers of neurons may exist to make a decision to call a group of pixels on an image normal or dysplastic.
Many recent studies have tested the possibilities of such computer aided diagnostics (CAD). The advantages that AI seems to bring to each endoscopy are the elimination of inter-observer or intra-observer variability in identifying abnormal lesions, combined with rapid, objective analysis. of all visual inputs in a consistent and fatigue-free manner. CAD could allow endoscopists to take targeted, high-yield biopsies in real time. Compared with random biopsies following the Seattle procedure or using advanced imaging, CAD can increase diagnostic efficiency and accuracy by limiting the risk of missed mucosal cancer. Furthermore, CAD can reduce risk by reducing the duration of secondary sedation by reducing procedure time.
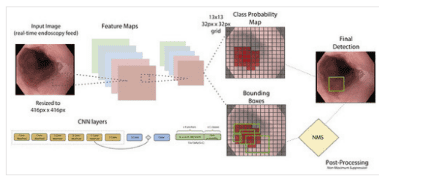
3.The role of computer aided diagnosis (CAD) systems in esophageal cancer diagnosis
Recent studies indicate that CAD can be successful in detecting cancerous lesions in patients with Barrett's esophagus. Von Der Sommen et al. have developed an ML algorithm that uses CAD to analyze texture and color in static images to detect early cancerous lesions in Barrett's esophagus.
Sensitivity and specificity are in the range of 0.90-1.00 and 0.65-0.91, respectively. In a study by Groof et al., six experts identified potentially cancerous tissues in the same image and used these expert-described images to train a computer algorithm to identify Barrett. neoplastic esophagus and non-malplastic Barrett's esophagus in the experimental cases. The resulting sensitivity and specificity of the computer algorithm are 0.95 and 0.85, respectively. Swager et al used CAD on ex vivo VLE imaging to retrospectively detect Barrett's esophagus and HGD without dysplasia or early adenocarcinoma. They were able to achieve a sensitivity of 0.90 and specificity of 0.93 while using the VLE as the reference image instead of high-definition white light endoscopy.
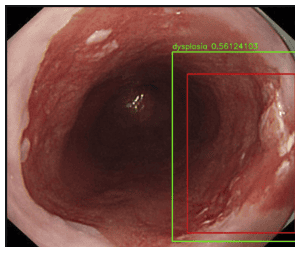
4. Disadvantages of artificial intelligence AI in diagnosing esophageal cancer from Barrett esophagus
Although the data are promising, nearly all studies have focused on training an algorithm on a set of retrospectively collected images. These studies are unfortunately subject to sample selection bias because images are often selected for high definition and are often from a single endoscopic center.
As a result, algorithms are often overtrained on a relatively small sample set and cannot generalize to other images of poorer quality or a population with low incidence and/or incidence. Barrett's esophagus is different. A small number of prospective or real-time studies currently exist and they were performed on a fairly small sample size. Furthermore, the standardization of AI systems is proving difficult, since the details of the algorithm are in a "black box" and inaccessible to direct reviews and revisions.
Difficulties encountered in using AI for mucosal identification Barrett has encountered in identifying early esophageal cancer. Although promising, the thresholds for early esophageal cancer detection below the American Gastrointestinal Endoscopy criteria may be secondary to limited imaging and lack of real-time imaging capabilities. Hashimoto et al may have found a way around previous difficulties by being able to create a faster algorithm that allows real-time video overlays using a large database of images. Using this technique, Hashimoto and his colleagues were able to identify early esophageal tumors with high accuracy.
5. Future AI Challenges
The process of standardizing ML algorithms poses a difficult challenge. Algorithms may be different for white light endoscopy than for NBI, VLE, or pCLE. It is possible that small differences such as the brand of the endoscope, wavelength of light, or white balance could affect the specificity or sensitivity of an algorithm under test.
There is no guarantee that a single algorithm will work both in high-prevalence groups of Barrett's esophagus and low-prevalence groups. Ideally, several algorithms should be prospectively tested and compared with the current gold standard of randomized biopsies in large, multicenter randomized clinical trials. Some of these studies are currently underway. User databases such as ImageNet or GastroNet contain labeled image samples to use for training and testing algorithms.
To date, the ML platforms used have been developed by endoscopists. A recent study published by Ebigbo et al used real-time AI to identify cancers in Barrett's esophagus and found that the AI system works in a similar way to an endoscopist. Such programs can also help train non-specialists as well as fellows in gastroenterology by providing real-time feedback, thus propagating more endoscopy specialists in a span of time. short time. Of course, endoscopists are not experts.
6. Conclusion
AI represents a renaissance in endoscopy, but not a revolution. The benefit may lie in the improved recognition of dysplastic and malignant tissue among amateur endoscopists or fellows in gastroenterology, as professional endoscopists have similar performance to AI. Generalizability, robustness of one or several algorithms applicable to different imaging modalities or diverse populations, and the ability to easily modify algorithms are current obstacles. need to be addressed before we can reliably use AI in endoscopic management of Barrett's esophagus.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.