This is an automatically translated article.
The article was written by Mr. Nguyen Tien Lung - Cell Therapy Block, Vinmec High Technology CenterApril 2020, RESTEM Biotechnology and Baptist Health South Florida nonprofit healthcare organization announced that three COVID-19 patients with acute respiratory distress syndrome are the first to receive treatment. Successful treatment with umbilical cord mesenchymal stem cells in the US.
1. Acute respiratory failure due to COVID-19
Acute respiratory failure is a condition in which the lungs suddenly fail to function properly for gas exchange, causing hypoxemia, with or without hypercapnia. In this trial, patients with acute respiratory failure due to COVID-19 received intravenous umbilical cord mesenchymal stem cells infusion.
The results showed that just a few days after the infusion, their oxygen requirements fell from 100% to less than 50%, accompanied by a significant decrease in blood levels of biomarkers that determine their status inflammation.
According to Dr. Guenther Khoehne, associate director and chief of the blood and marrow transplantation unit of the Miami Cancer Institute, the remarkable ability of these cells is to reduce inflammatory processes, thus being a powerful tool. promise for patients with COVID-19 as well as other diseases. These patients improved their lung condition much faster than patients treated with other experimental therapies.
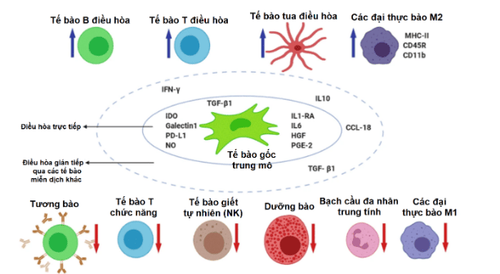
Hình 1. Cơ chế điều hòa giảm viêm của tế bào gốc trung mô
Mesenchymal stem cells secrete substances such as interleukin-1 (IL-1), IL-6, hepatocyte growth factor (HGF), Prostaglandin E2 (PGE2),... for direct regulation. , or indirectly through other immune cells, thereby activating regulatory T cells, regulatory B cells, M2 macrophages, as well as inhibiting functional T cells, functional B cells function, neutrophils.
This is the result of an emergency authorized test by the US Food and Drug Administration (FDA). With those positive results, the FDA approved a phase 1/2a clinical trial called SUCCESS (The Systemic Umbilical Cord Cells to Ease Severe Syndrome with COVID-19) to evaluate the use of mesenchymal stem cells. umbilical cord in the treatment of patients with acute respiratory distress syndrome due to COVID-19.
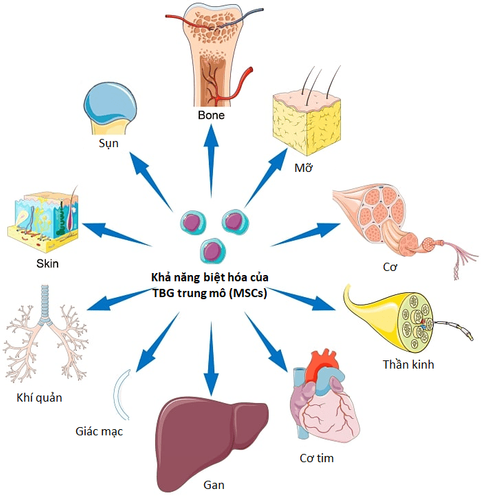
This trial will be carried out on 60 patients, to see if using this cell type can be a safe and effective treatment for critically ill COVID-19 patients. Mesenchymal stem cells (MSCs) are mature stem cells that have the ability to self-proliferate and differentiate into cells of connective tissue such as fat, bone, cartilage, and some cell types. other cells such as nerve cells, liver, pancreas, kidney....
In addition, they also secrete active substances that nourish cells, regenerate blood vessels, anti-inflammatory factors, prevent cell death and prevent cell death. immunomodulatory ability.
Umbilical cord mesenchymal stem cells isolated from the umbilical cord of newborns have many advantages such as: the ability to multiply in large numbers in the laboratory, low immunogenicity and high immunomodulatory ability As a result, this cell type is promising for the treatment of acute and chronic diseases, including acute respiratory failure and many other diseases related to COVID-19.
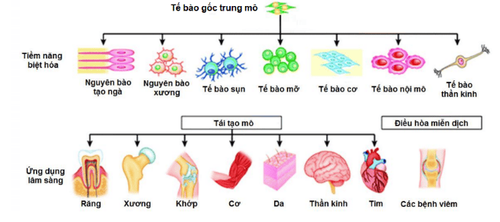
Hình 2. Tiềm năng ứng dụng của tế bào gốc trung mô
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.