This is an automatically translated article.
Articles by Dr. Hoang Quoc Chinh - Cancer geneticist and Dr. Nguyen Hong Thanh - Researcher, Vinmec High-Tech Center
Oncologic fluid biopsy refers to the isolation and analysis of tumor-derived materials such as DNA, RNA, intact cancer cells, and extracellular vesicles (EVs). ) in body fluids such as blood, urine, saliva, and feces [1,2]. Solid tumors release a variety of materials into body fluids at nearby tissue sites, and most commonly fragments of tumor-derived DNA (ctDNA) or tumor cells (CTCs). circulating in the blood.
The analysis of free DNA fragments in the blood has been used frequently in other routine tests such as prenatal screening tests for Down syndrome [3]. The liquid biopsy approach is also increasingly being applied in the management of cancer patients, especially those with metastatic cancer. Liquid biopsy technique has the essence of using body fluids such as blood, urine, and saliva, so it is non-invasive and can be used repeatedly when needed and has the ability to be used for early detection. Cancer, diagnosis, prognosis, prediction of response to treatment, and monitoring of cancer metastasis. This review will primarily discuss the role of liquid biopsy in cancer diagnosis and screening.
Xét nghiệm sàng lọc trước sinh đối với hội chứng Down
cfDNA is released into the bloodstream through apoptosis or cell necrosis, first discovered in 1948 [4]. cfDNA is rapidly cleared from the systemic circulation, usually within an hour [5]. In cancer patients, a very small fraction of cfDNA [6,7] consists of DNA derived from apoptosis or necrosis (ctDNA) and ctDNA is mainly distinguished from cfDNA by specific features. specific molecular scores such as mutations, copy number variations, methylation changes, or tumor-associated integrated viral sequences [8]; ctDNA has been shown to reflect mutational markers of the primary tumor [9].
A wide range of assay methods with different limits of detection (LoDs) are used to detect ctDNA and they include quantitative PCR, digital PCR (Droplet Digital PCR, BEAMing - Beads, Emulsions, Magnetic and Amplification), targeted gene sequencing (gene panel) or whole genome sequencing (whole genome sequencine) using molecular barcode marking [7,10,11] . Currently, digital PCR-based methods appear to be the most sensitive and relatively low cost with the ability to detect known mutations with extremely low allele frequencies (0.01%) in cfDNA. Potential applications of ctDNA analysis include:
1) detection of residual cancer cells (MRD); 2) predict the risk of recurrence and monitor metastatic tumors e.g. in breast cancer [12–15] and diagnostic testing for EGFR mutations. These tests have now been approved by the US Food and Drug Administration (FDA) [16].
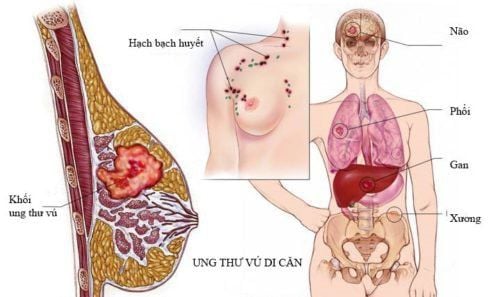
Các ứng dụng tiềm năng của việc phân tích ctDNA dự đoán nguy cơ tái phát và theo dõi các khối u di căn như ung thư vú
EVs or exosomes or secretory bodies are circular vesicles 30–120 nm in diameter that carry genetic material including DNA, mRNA, microRNA (miRNA) and a number of proteins that act as regulatory mediators. interactions between cells [24]. Cancer-derived EVs contain different cancer-associated molecules [25] and have been shown to reflect MRD status as well as predict response to therapy [26].
Free miRNAs are non-coding RNA molecules [19–24] with varying degrees of expression depending on the type of cancer [27]. The stable properties of these molecules make them an attractive biomarker for liquid biopsy-based assays.
In addition to being non-invasive and therefore easy to repeat, liquid biopsies have several other unique advantages over conventional cancer screening and early detection methods as well as solid tissue biopsies for cancer diagnosis . The first and foremost advantage is the ability to obtain complete information regarding tumor heterogeneity. Tumor heterogeneity, clone evolution and their impact on treatment response and outcome are increasingly recognized [28-31].
This is a very important thing in making an accurate diagnosis and providing the right treatment regimen for each specific biomarker. The collection of information on tumor heterogeneity and molecular biomarkers helps in early detection and differentiation of benign and malignant tumors [34] and enables detection of different types of cancer. cancer is potentially lethal, thus minimizing overtreatment.
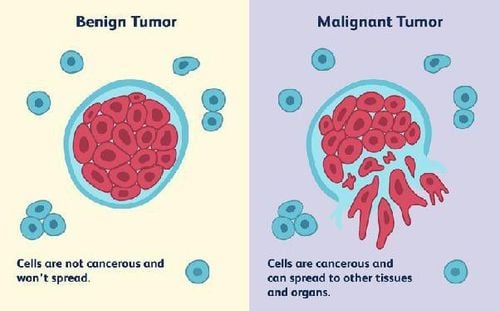
Hình ảnh phân biệt khối u lành tính và khối u ác tính
Overdiagnosis or overdiagnosis is a controversial issue for cancer screening services. Overdiagnosis is primarily caused by the detection of precancerous lesions (eg, ductal carcinoma in situ) or cancers that are never life-threatening. These cancers usually release less material into the blood vessels. Thus, theoretically, there should be minimal or no release of tumor material into the bloodstream. Thus, screening based on liquid biopsies can help reduce overdiagnosis. However, this claim requires specific scientific evidence.
Technologies used to detect biomarkers such as free DNA (ctDNA) or tumor free cells (CTCs) in liquid biopsies are of rapidly evolving interest, especially with improvements in sequencing technology, image analysis, and computational techniques. Much of the current research on early detection focuses on ctDNA. Because CTCs and ctDNA have complementary informational value [23] and assays with free tumor cells are increasingly sensitive, the combined detection of CTCs and ctDNA has ability to improve both sensitivity and specificity. Furthermore, morphological information and genomic data [18] obtained from CTCs can help inform further imaging indications by locating the latent cancer. Studies are underway to develop cfDNA-based genomic atlas [41,43] to address the problems of false positives.
Thus, liquid biopsy has significant potential for early detection and diagnosis of cancer. Research is underway to resolve these controversial issues in order to exploit the full potential and application of tests using liquid biopsies.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.