This is an automatically translated article.
Article by Doctor, Doctor Hoang Minh Duc - Vinmec Stem Cell Research Institute and Gene Technology
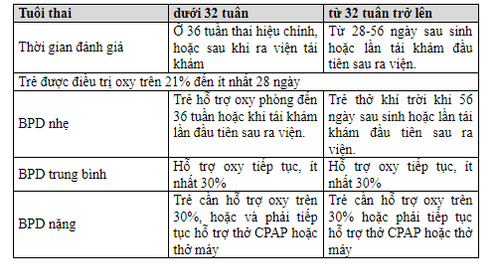
Bronchopulmonary dysplasia (LSPQP) is defined as a chronic lung disease that occurs when an infant requires oxygen support at least up to 28 days after birth, the severity depends on the degree of oxygen dependence, mechanical ventilation support, in order to Maintain SpO2 above 89%.
For children from 32 weeks, the time to determine chronic lung is from 28 to 56 days after birth. For infants before 32 weeks, the time to determine pulmonary fibrosis is 36 weeks of corrected gestational age.
1. Classification of bronchopulmonary dysplasia in neonates
Worldwide, it is estimated that more than 1 in 10 babies are born prematurely, every year about 15 million babies are born prematurely, of which more than 1 million children die. Bronchopulmonary dysplasia is the most common complication in premature infants, accounting for 5–68%. Progressive bronchopulmonary dysplasia is a consequence of maternal preeclampsia, pleurisy, postpartum mechanical ventilation, high oxygen levels, and many other risk factors.
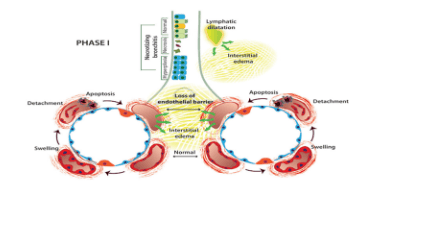
Bronchopulmonary dysplasia is defined when the infant is oxygen dependent up to 36 weeks of corrected gestational age. The disease occurs as a result of high oxygen levels, trauma caused by ventilatory pressure causing trauma to the developing lung cells, inhibiting and reducing the growth of alveoli and blood vessels. Direct injury to cells occurs due to an inflammatory reaction between oxidant and antioxidant factors. The pathogenesis of pulmonary dysplasia is caused by a combination of factors, in which inflammatory response is the main factor and is divided into 3 stages:
Stage 1 (acute): + within 4 days: Edema of interstitial tissues , endothelial barrier is broken, bronchial epithelial necrosis, maintenance of fetal circulation.
Stage 2: Fibrosis of interstitial tissue, dilation and collapse of bronchi, beginning of thickening of bronchial epithelium due to bronchioles II, necrosis of bronchi, overgrowth of smooth muscle layer. fetal circulation, reduced blood supply capillaries
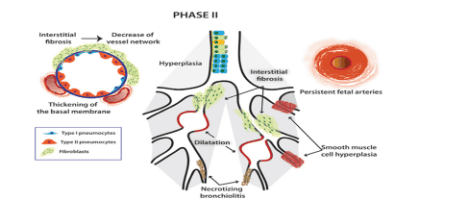
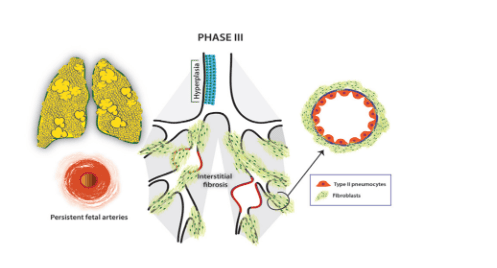
Stage 3: Fibrosis of interstitial tissues increases, air retention, alveolar collapse, bronchial collapse, fetal circulation persists.
Pathophysiological risk factors affecting lung developmental stages are shown in the diagram above.
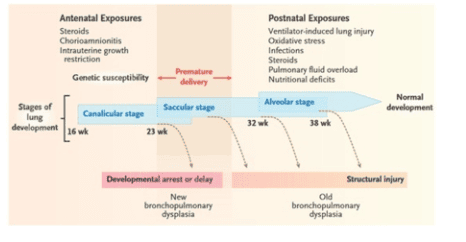
Immature/premature lungs: Vulnerable to oxygen damage, gas trauma, lack of surfactant, poor resistance to oxidants; Oxygen poisoning (via free oxygen radicals); Trauma to gas, pressure (ventilator); Pulmonary edema (due to a lot of fluid and ductus arteriosus); Respiratory infections (nosocomial bacteria); Impaired ability to synthesize surfactant (late); Deficiency of copper (Cu), zinc (Zn), manganese (Mn) increases the risk of lung injury; Vitamins A and E should not be prevented (preserving cells, preventing lipid degradation); Genetic factors (Genes); Because the mother has amnionitis, do not use antenatal steroids, preterm placental abruption, and Indomethacin before birth.
2. Application of mesenchymal stem cells in the treatment of bronchopulmonary dysplasia
In 2013, Ahn and colleagues performed mesenchymal stem cell transplantation from umbilical cord blood in a mouse model of lung injury due to hyperoxygenation. The results showed that stem cell transplantation increased alveolar numbers, decreased inflammatory response, reduced pulmonary vascular injury, decreased fibrosis, and decreased pulmonary arterial hypertension. A meta-analysis of preclinical studies in animal models for Sajit's bronchopulmonary dysplasia was conducted in 2017 (25/990 eligible studies). The results showed that mesenchymal stem cells are safe and reduce pulmonary hypertension, pneumonia, reduce fibrosis, increase number and improve vascular structure.

In 2013, Yun Sil Chang et al conducted cord blood stem cell transplantation on 9 premature infants at risk of bronchopulmonary dysplasia with gestational age of 25.3±0.9 weeks, weight 793± 127-gram on day 10.4± 2.6 postpartum. Nine children were divided into two groups: low dose (3 patients with 1x107 cells/kg) and high dose 2x107 cells/kg. Research results show that the procedure is safe, without complications. The percentage of children with severe pulmonary fibrosis was significantly reduced in the group receiving the MSC pump and there was no clear difference between the 2 groups. Powell in 2019 also announced the results of endotracheal cord blood stem cell transplantation (allogeneic) for the treatment of bronchopulmonary dysplasia. The results also showed safety (no SAE was recorded) and feasibility of the therapy. According to a report on the clinicaltrials.gov website, three studies are also in the enrollment phase. Specifically:
Won-soon Park is conducting a phase II, randomized controlled clinical trial on 70 endotracheal cord blood stem cell transplant patients (2014-2020). The study is currently in the patient selection phase. Maria-Spain (February 2019) is also recruiting LSPQP patients to carry out a venous TBG transplant but the origin is unknown. Bai-Horng Su-Taiwan (2010) conducted a phase I randomized, controlled clinical trial on 10 patients with LSPQP. Endotracheal TBGTMDR transplantation was performed but the outcome is unknown. In Vietnam, Vinmec International General Hospital conducted central intravenous infusion and endotracheal infusion of autologous stem cell mononuclear cells from bone marrow to treat a 30-week-old newborn with bronchopulmonary dysplasia in 2015. The results showed that the patient was weaned from oxygen after 1.5 months of stem cell transplantation, the structure and function of the lung improved markedly.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.