This is an automatically translated article.
Posted by Doctor Mai Vien Phuong - Department of Medical Examination & Internal Medicine - Vinmec Central Park International General Hospital.
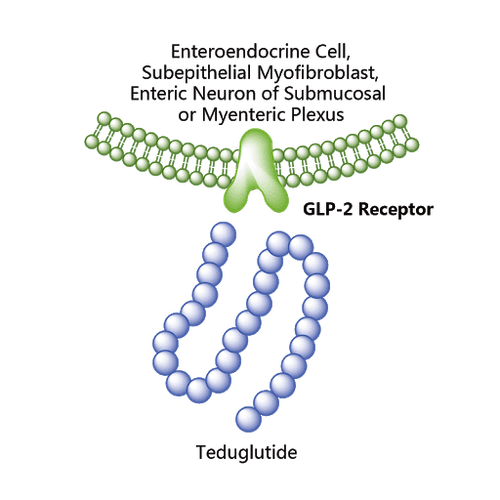
Teduglutide, a recombinant, anti-aging, longer-acting GLP-2 analog, was shown in an open-label study to be safe, well tolerated, gut stimulant, and significantly increase absorption Wet intestinal absorption, but no energy absorption, in 16 short bowel syndrome patients with jejunostomy or colostomy.
1. The role of Teduglutide in parenteral nutrition
Teduglutide, a recombinant, anti-aging, longer-acting GLP-2 analog, was shown in an open-label study to be safe, well tolerated, gut stimulant, and significantly increase absorption Wet intestinal absorption, but no energy absorption, in 16 short bowel syndrome patients with jejunostomy or colostomy. Teduglutide was subsequently studied in two multinational, randomized, double-blind, placebo-controlled phase III trials including a total of 169 patients with short bowel syndrome with parenteral or parenteral nutrition in outpatients. reside. Patients in both trials followed a habitual diet. Notably, only about half of the subjects used anti-diarrheal and anti-secretory drugs during the study.
2. The role of Teduglutide in increasing muscle mass
In the first study, 83 short bowel syndrome patients were divided into 3 treatment groups (placebo, 0.05 mg/kg/day and 0.10 mg/kg/day subcutaneously once daily) and were treated with study drug for 6 months after the optimized parenteral nutrition period.
The main endpoint of parenteral nutrition was the duration of the reduction in parenteral nutrition (20% reduction at week 20 and maintenance until week 24). Teduglutide has been shown to be safe and well tolerated; However, there was a strong trend toward overall volume reduction at the end of treatment in the teduglutide group compared with placebo (2.5 L/week versus 0.9 L/week, respectively). ;P = 0.08). Sugar energy intake, although much lower than baseline, was not significantly different from placebo at the end of 24 weeks of treatment (P=0.11). Plasma citrulline concentrations and lean body mass were significantly increased in the teduglutide group compared with placebo; No evidence of metaplasia in intestinal samples was detected.
3. The role of Teduglutide in reducing the amount of fluid required for parenteral nutrition therapy
Second trial comparing a lower dose of teduglutide with placebo administered for 6 months in 86 adult short bowel syndrome patients and using a more aggressive parenteral nutrition withdrawal strategy (10-30 % reduction vs. with 10% over a 2 week period and starting at week 2 versus week 4.
Again, a significant benefit of teduglutide over placebo was seen.Teduglutide users were more likely to respond to therapy was more than doubled (63% vs 30%, P = 0.02) The mean reduction in enteral nutrient volume after 24 weeks was 4.4 L in the teduglutide group compared with 2.3 L in the teduglutide group. Fifty-four percent of those taking teduglutide experienced a reduction of at least 1 parenteral nutrition per day/week compared with 23% for placebo. At the end of 24 weeks of treatment
In a preliminary report from a 2-year study, 65 patients (74%) completed the study Out of 30 patients treated for 30 months With teduglutide, 28 (93%) significantly reduced parenteral support with a mean reduction of 7.6 L/week and 21 (70%) eliminated at least 1 day of infusion. A total of 15 of the 134 (11%) patients treated in both the phase III studies and their extension achieved bowel autonomy. Most of these patients had a partial need for colostomy and lower baseline parenteral nutrition/IVF requirements. Because the numbers are so small, it is not possible to perform formal statistical analysis for the predictors.
4. Teduglutide side effects
The most common gastrointestinal side effects reported in both trials included abdominal pain, nausea, stomatal changes (in those undergoing hysterectomy), abdominal distension and peripheral edema; Resolution occurs with continued treatment or temporary discontinuation in most cases. Data from extensive studies suggest an acceptable safety profile with abdominal pain, injection site reactions, and stomach complaints being the most common. Although anti-teduglutide antibodies have been demonstrated in the blood of treated patients, they appear to be non-neutralizing and have not been shown to have an effect on reducing the volume of parenteral nutrition. Therefore, it appears that long-term teduglutide treatment is associated with acceptable tolerability and continued improvement. On the basis of the data from these two important tests,
5. Contraindications, Precautions and Costs associated with the use of Teduglutide
The only contraindication to the use of teduglutide is gastrointestinal neoplasia. In patients with active, non-GI neoplastic disease, use should only be considered if the benefits outweigh the risks. However, caution should be exercised due to some possible side effects including increased fluid absorption and the potential for fluid overload; The potential for increased absorption requires dose reduction and drug monitoring when using drugs with narrow therapeutic windows or titration requirements (eg, benzodiazepines, opioids, psychotropic drugs) and the risk of accelerated tumor growth. in the intestine requires periodic endoscopic monitoring before and during its use (6 months before, 1 year later, and at least every 5 years thereafter).
Additional monitoring for gastrointestinal obstruction, gallbladder, biliary and pancreatic disease (amylase, lipase, alkaline phosphatase and total bilirubin before and every 6 months during use) is also recommended as part of the treatment. risk assessment and mitigation strategy (REMS-https://www.gattex.com/hcp/rems.aspx) mandated prescriber program).
6. Cost of Teduglutide Treatment in the US
Given the average annual cost of $295,000 associated with the use of teduglutide in the United States, appropriate patient selection will be critical to positioning this therapy appropriately in patient management parenteral nutrition - short bowel syndrome.
Note, in the US, the cost to the individual is generally much lower because there are insurance and patient assistance programs that provide financial assistance for out-of-pocket expenses. Another interesting predicament that may need to be considered, especially in patients who have completely stopped from supportive parenteral nutrition while on teduglutide, there may be a potential for insurance to deny continued coverage. continued use of teduglutide because the patient was no longer on parenteral nutrition. It is important to realize that the reduction in costs associated with the use of parenteral nutrition when parenteral nutrition is discontinued will partially offset the costs associated with the use of teduglutide. Finally, some clinically important outcomes that defy calculation can come in the form of significantly improved quality of life due to reduced fecal intake, preserved liver function due to less external nutritional dependence. intestines and even avoid a bowel transplant.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References
Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 1999;117:1043-1050. Amiot A, Messing B, Corcos O, et al. Determinants of home parenteral nutrition dependency and survival of 268 patients with non-malignant short bowel syndrome. Clin Nutr 2013;32:368-74. Byrne TA, Persinger RL, Young LS, et al. A new treatment for patients with short-bowel syndrome: growth hormone, glutamine, and a modified diet. Ann Surg 1995;222:243-254. Byrne TA, Cox S, Karimbakas M, et al. Bowel rehabilitation: an alternative to long-term parenteral nutrition and intestinal transplantation for some patients with short bowel syndrome. Transplant Proc 2002;34:887-890.