This is an automatically translated article.
The article is written by MSc, BS. Mai Vien Phuong, Department of Medical Examination & Internal Medicine - Vinmec Central Park International General HospitalGastric cancer (GC) is the third leading cause of death from malignancy worldwide, and gastroduodenal endoscopy (EGD) is considered the best diagnostic tool for cancer. in the early stages and artificial intelligence plays an important role in the treatment of stomach cancer.
Artificial intelligence (AI) is based on artificial intelligence elements that perform functions related to the human mind, such as learning and problem solving.
In colonoscopy, AI has begun to aid in improving colon polyp detection and adenoma detection (ADR) rates, to distinguish between benign and precancerous lesions based on the interpretation of patterns their surface.
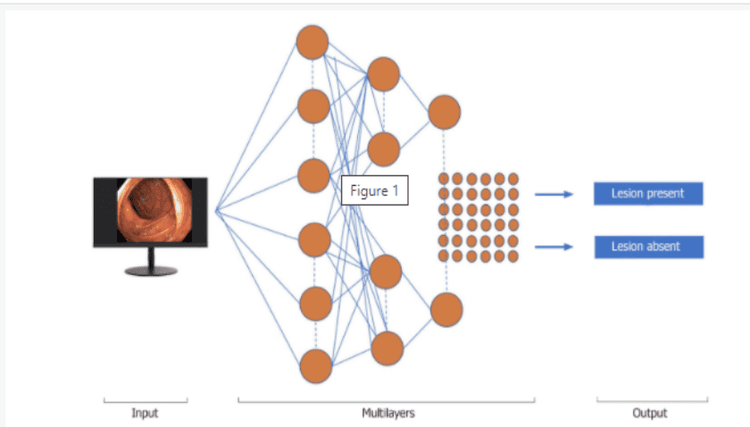
Machine learning (ML) and deep learning (DL) can be considered as subfields of artificial intelligence. ML is a form of artificial intelligence that can support decision-making, allowing improvements to applied algorithms without programming, including examining data and implementing descriptive models and models. predicted (Figure 1).
1. Artificial Intelligence and Stomach Cancer
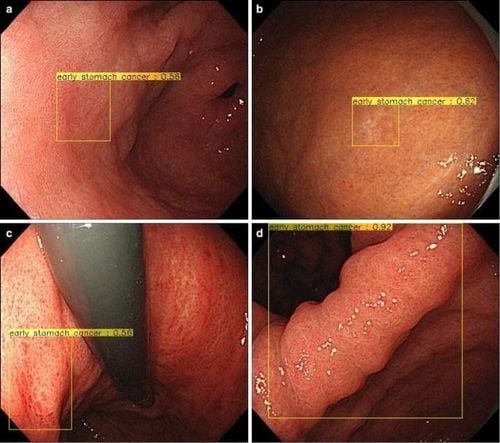
Gastric cancer (GC) is the third leading cause of death from malignancy worldwide, and gastroduodenal endoscopy (EGD) is considered the best diagnostic tool for cancer. at the early stage. The treatment of gastric tumor depends on the depth of the submucosal invasion; indeed, for differentiated intramucosal tumors (M) or those that invade the superficial submucosa (≤ 500 lm: SM1), EMR mucosal resection or submucosal dissection ESD is performed to resect the lesion, while tumors with deep submucosal invasion (>500 lm:SM2) should be treated surgically to avoid the potential risk of local invasion and metastasis. Magnified endoscopy in combination with NBI or FICE (flexible color enhancement of spectral imaging) is clinically useful in differentiating gastric malignancy from non-malignant regions. However, this optical diagnosis is completely dependent on the expertise and experience of the operator, which precludes its general use in clinical practice.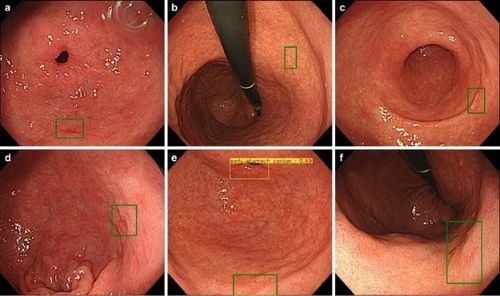
2. Results of some studies
Two multicenter randomized studies examined the performance of endoscopy with/without the assistance of AI algorithms. The first study estimated the performance of a real-time DL system, WISENSE, to control for the presence of blind spots during upper gastrointestinal endoscopy. Overall, 324 patients who underwent random endoscopy with or without the WISENSE blind spot monitoring system with an average accuracy of 90% and a site-specific accuracy ranging from 70.2% - 100% in 107 live endoscopic videos.Mean sensitivity and specificity were 87.6% and 95%, and ranged from 63.4%-100% and 75%-100%, respectively. For the timing endoscopic procedure, the WISENSE system accurately predicted the start and end times in video by 93.5% (100/107) and 97.2% (104/107), respectively.
Miyaki et al., have developed software that allows quantitative assessment of mucosal GC on magnified gastrointestinal endoscopy images obtained with FICE. They adopted a feature framework featuring densely sampled scale-invariant feature descriptors to magnify the FICE images of 46 cases of intramucosal gastric cancer and then compared with histological findings. The CAD system enables detection accuracy of 86%, sensitivity and specificity of 85% and 87% for cancer diagnosis.
In the study by Kanesaka et al., a total of 127 patients with gastroscopy contributed to 127 cancerous M-NBI images, while 20 patients without gastroscopy contributed to 60 M-NBI images. NBI is not cancerous. The authors have created software that enables the identification of both gastric cancer and demarcation between malignant and non-malignant regions. This CAD algorithm was designed to investigate the gray-level co-occurrence matrix features of the partitioned pixel slices of magnified NBI images, and a support vector machine was used for the machine learning approach. Models showed a sensitivity of 97% and specificity of 95% in discriminating cancer, while the performance for region concordance displayed sensitivity and specificity, of 81% and 66%, respectively.
3. Applications of complex neural networks CNN in gastric cancer assessment
In 2018, Hirasawa et al., developed an AI-based diagnostic system to detect stomach cancer, using CNN that simulates the human brain.A total of 714 out of 2,296 laboratory imaging sets (31.1%) confirmed the presence of gastric cancer, and 84.1% had moderate/severe gastric atrophy. CNN used 47 seconds to analyze 2,296 laboratory images, diagnosing 232 patients with gastric cancer overall, 161 non-malignant lesions, 71 out of 77 gastric cancer lesions with a sensitivity of 92, 2%. The majority of gastric lesions (98.6%) with a diameter of ≥6 mm were correctly identified by CNN, in addition to all invasive carcinomas (T1b or deeper). Undiagnosed lesions have a concave surface and are often endothelial carcinoma with differentiated tissue, which distinguishes from gastritis is also a challenge for experienced endoscopists. Another common reason for misdiagnosis is the anatomical locations of the myocardium, pylorus, and pylorus.
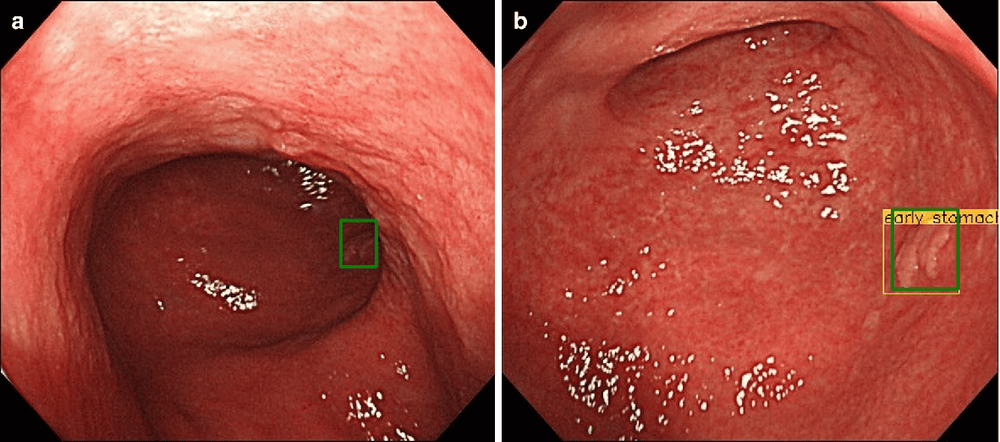
A total of 790 endoscopic images of gastric cancer were used for the machine learning algorithm, while 203 additional images, completely autonomous from the learning literature, were processed as a test set. The AI model showed a sensitivity and specificity of 76% and 96%, respectively, in discriminating SM2 or deeper cancer invasion, with a higher diagnostic performance than the model shown by the endocrinologists. can be achieved. This high specificity could reduce the overestimation of tumor invasion, which would indirectly contribute to a reduction in avoidable surgeries for M/SM1 melanoma. Furthermore, in this study, the CNN-CAD system also achieved significantly higher accuracy and specificity than both the professionally trained and junior endoscopists.
4. The role of AI in predicting the prognosis of patients with stomach cancer
AI can help doctors predict the prognosis of patients with stomach cancer. Several important clinical trials evaluating adjuvant strategies for highly advanced gastric cancer have been produced in the past decade, but the most appropriate therapy for gastric cancer to date remains uncertain. . In addition, two contemporary molecular landscape studies have demonstrated the presence of different molecular gastric cancer subtypes. A deep learning algorithm-based model (survival recurrence network, SRN) was developed to predict survival events for a total of 1190 gastric cancer patients, based on clinical/pathological data as well as as treatment protocol, predict outcomes at each time point during a 5-year monitoring period. The SRN indicates that the mesenchymal subtype of gastric cancer should stimulate an appropriate postoperative treatment strategy as a consequence of the enormous recurrence rate. In contrast, the SRN observed that microscopic and papillary-type unstable gastric cancers showed a significantly more favorable prognosis after chemotherapy including capecitabine and cisplatin. SRN achieved a survival rate of 92%, 5 years after gastrectomy. The ANN model was used to evaluate 452 gastric cancer patients, determined survival with an accuracy of about 90%, and focused on creating an ANN structure suitable for data processing. whether censored. Specifically, 5 sets of unique time point transition ANN models were generated to predict gastric cancer patient outcomes at regular intervals (yearly) up to the fifth year after resection. stomach . Therefore, ANN prediction models exhibit accuracy, sensitivity, and specificity, 88.7%-90.2%, 70.2%-92.5%, and 66.7%, respectively- 96.2%.5. Some limitations of artificial intelligence
Artificial intelligence AI could represent an essential diagnostic method for endoscopists and gastroenterologists to treat patients accordingly and predict their clinical outcomes. Artificial intelligence appears to be particularly valuable in gastrointestinal endoscopy, to improve the detection of premalignant and malignant lesions, or inflammatory lesions, GI bleeding, and pancreatic pathologies. However, the current limitations of Artificial Intelligence include the lack of high-quality data sets for developing ML machine learning algorithms. Furthermore, an important piece of evidence used to build machine learning algorithms has only come from preclinical studies. Certainly, there is also a need to acknowledge the ethical issues as the AI is not aware of the patient's choices or liabilities. Privacy issues can be solved using linked datasets that do not involve centralized servers. Future randomized studies may directly increase the overall value (quality versus cost) of CNN by examining its impact on surveillance colonoscopy, colonoscopy duration, polyps and ADRs, and pathological costs. As the science of AI is in development, current limitations must be considered as future challenges, so they are truly inherited in medical applications, including the ability to predict difficult to situations characterized by uncertainty. All in all, AI is revolutionizing technology and impacting other ethical aspects like replacing human work with machines, but this has always been an open question since the industrial revolution. What can be done is to foster mutual cooperation through gastrointestinal endoscopy applications, to mutually benefit from achievements in both scientific fields.Stomach cancer is a dangerous disease, common in Vietnam and very life-threatening. In order to prevent and detect stomach cancer early, you should treat stomach ulcers early and regularly screen for stomach cancer to proactively detect the risk of early disease at an early stage, bringing effective treatment results. best treatment.
Currently, Vinmec has a SUPPORT PACKAGE FOR THE TREATMENT OF Stomach Ulcers including functional foods to help prevent and support comprehensive treatment. The product packages are designed according to medical standards, ensuring safety and providing comprehensive treatment effects for customers. Each product in the package is subjected to strict, precise input quality control, which has a mutual effect. The dosage and route of oral administration are designed to ensure just enough to maximize the effect, on the other hand, do not cause excess/deficiency or chemical reactions in the products together. Customers not only receive advice from pharmacists and support from well-trained and highly qualified doctors if their needs arise during use, but also detailed and scientific instructions on how to use them. how to drink as well as the dose according to each meal.
In addition, Vinmec also offers a package of screening and early detection of cancers of the gastrointestinal tract (esophagus - stomach - colon) combined with clinical and paraclinical examination to bring the most accurate results possible. If you have a need to examine and use service packages at Vinmec, please register to book an appointment at the website or contact Vinmec's hotline system for detailed advice.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
Reference1. Russell S, Norvig P. Artificial Intelligence: A Modern Approach, Global Edition. 3rd editon. London: Pearson, 2016.
2. Colom R , Karama S, Jung RE, Haier RJ. Human intelligence and brain networks. Dialogs Clin Neurosci . 2010; 12 :489-501. [PubMed]
3. Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst . 2005; 97 :142-146. [PubMed] [DOI]
4. Gaetano Cristian Morreale, Emanuele Sinagra, et al., Emerging artificial intelligence applications in gastroenterology: A review of the literature, Artif Intell Gastrointest Endosc. Jul 28, 2020; 1(1): 6-18