1. What is Transmetil?
Each Transmetil tablet contains Ademetionine 500 mg.
Ademetionine is a derivative of the amino acid methionine, also known as S-adenosylmethionine (SAMe), a key intermediate metabolite of methionine. This active substance participates in methylation processes essential for cellular growth and repair, maintaining the phospholipid bilayer of cell membranes. Ademetionine is naturally present in the brain and liver, where it supports neurotransmitter activity, hormone regulation, and mood stabilization.
Under normal conditions, the body synthesizes sufficient Ademetionine to maintain good health. However, deficiencies in methionine, folate, or vitamin B12—commonly associated with cholestatic liver diseases—can lead to decreased Ademetionine levels. Patients with such deficiencies require supplementation with pharmaceutical forms of this compound, as Ademetionine does not naturally occur in food.
S-adenosylmethionine is not only crucial for the immune system but also for maintaining cell membranes and producing brain chemicals such as serotonin, dopamine, and melatonin.
2. Indications for Transmetil
Transmetil is indicated for the treatment of the following conditions:
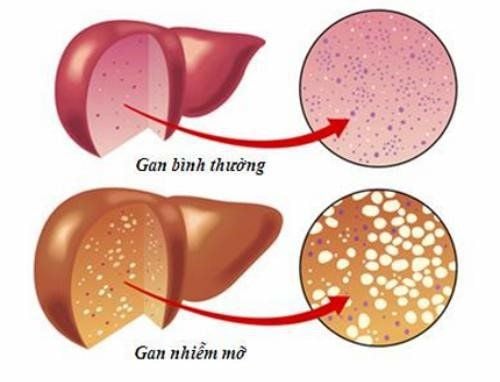
- Cholestasis in cirrhosis or precirrhotic states.
- Intrahepatic cholestasis during pregnancy.
- Management of depressive symptoms.
Transmetil should not be used in the following cases:
- Hypersensitivity to any component of the drug.
- In children, unless deemed absolutely necessary.
3. Dosage and Administration of Transmetil
3.1. Administration
Transmetil is administered orally. Patients should swallow the tablets whole with half a glass of water, without breaking, crushing, or chewing them, as this could reduce the drug's absorption. Transmetil should not be taken with food. Initial therapy may begin with intramuscular or intravenous injections before transitioning to oral administration, or it may start with oral use directly.
3.2. Dosage
Treatment of cholestasis in cirrhosis or precirrhosis:
- Initial therapy: Intramuscular or intravenous injections at 5–12 mg/kg/day for the first two weeks.
- Maintenance therapy: Oral administration of 10–25 mg/kg/day.
Treatment of depressive symptoms:
- Initial therapy: Intramuscular or intravenous injections of 400 mg/day for the first 15–20 days.
- Maintenance therapy: Oral administration of 800–1200 mg/day.
Pediatric use: The safety and efficacy of Transmetil in children have not been established.
Elderly patients: It is recommended to start at the lowest possible dose within the therapeutic range.
4. Side effects of Transmetil
Patients using Transmetil 500 mg may experience the following side effects:
- Common side effects: Gastrointestinal disturbances such as nausea, abdominal pain, or diarrhea.
- Other side effects: Skin and subcutaneous tissue disorders, neurological disturbances, fatigue, injection site reactions, and musculoskeletal disorders such as joint pain, muscle stiffness, or peripheral edema. Patients experiencing any symptoms suspected to be side effects due to taking. Transmetil should notify their treating physician for safe and timely treatment advice.
5. Drug Interactions of Transmetil
Transmetil may interact with the following drugs or substances:
- Selective serotonin reuptake inhibitors (SSRIs).
- Tricyclic antidepressants
- Over-the-counter products containing Tryptophan.
- Herbal medications
Patients should inform their doctor about the medications or dietary supplements they are taking for advice and consideration before starting treatment with Transmetil to avoid interactions that affect the effectiveness of the drug.
6. Precautions when using Transmetil
- Patients with pre-cirrhosis and cirrhosis need to have their ammonia levels controlled during treatment.
- Ademetionine should not be used in patients with bipolar disorder because of the possibility of transitioning from depression to hypomania or mania
- Caution is advised when using Transmetil in elderly or renal-impaired patients.
- Serotonin syndrome may occur in patients taking Ademetionine concurrently with Clomipramine.
- The short-term efficacy of Transmetil in treating depression (3–6 weeks) has been demonstrated, but long-term efficacy remains unstudied.
- Psychological support should accompany the treatment of depression with Transmetil.
Patients should avoid operating machinery, driving, or engaging in activities requiring focus until assured that the drug does not impair their abilities. - Pregnant women: Transmetil may be used at higher doses in the third trimester. However, during the first trimester, the benefits and risks should be carefully weighed before commencing treatment.
- Breastfeeding women: The excretion of Transmetil into breast milk has not been studied; therefore, caution is advised.
- Do not use the drug if the tablets show signs of melting, discoloration, altered odor, or expired.
The above is comprehensive information on Transmetil. Patients should carefully read the instructions on the packaging, consult a physician, and strictly adhere to prescribed indications and dosages for optimal effectiveness.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.