This is an automatically translated article.
Post by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General Hospital
The choice of treatment modality for bleeding ulcerative colitis depends on the severity, extent of the lesion and individual characteristics of each patient such as frequency of recurrence, course of disease, response to drugs. previous treatment, side effects during treatment. This article talks about how to treat ulcerative colitis with immunosuppressants
1. Treatment of bleeding ulcerative colitis with immunosuppressants
Cyclosporine
The effectiveness of Cyclosporine in cases of severe bleeding ulcerative colitis that has failed steroids has been demonstrated in clinical trials with treatment response rates ranging from 76 to 85%. The dose of 2mg/kg/day is currently used as the standard dose in initiation of treatment and the rate of surgery at different points in hospital stay, within 3 months, 12 months is 25%, 30% and 30%, respectively. 45%. Time to assess response to treatment is after 4 days. However, due to narrow indications for treatment as well as concerns about undesirable effects, in practice, the proportion of patients treated with cyclosporine is not high. According to a national IBD study in the UK, only 24% of patients with steroid-resistant severe bleeding ulcerative colitis were treated with cyclosporine. For patients treated with cyclosporine who were successfully converted to maintenance oral thiopurine, the incidence of surgery at longitudinal follow-up was significantly reduced. However, in patients who have failed prior thiopurine therapy, cyclosporine should not be selected as salvage therapy.
Tacrolimus This is a calcineurin inhibitor with a mechanism similar to cyclosporine. In a meta-analysis and review study, the clinical response after 2 weeks was significantly higher in the tacrolimus group than in the placebo group. The rate of no surgery at 1 month, 3 months, 6 months and 1 year was 86%, 84%, 78% and 69%, respectively.

Infliximab The use of infliximab (IFX) as a single dose of 5 mg/kg is an effective salvage therapy in severely steroid-resistant patients. A randomized controlled trial reported a significantly lower 3 month surgical rate compared with placebo in patients initially treated with intravenous bethamethasone but no response (7/24 vs 14/21, p<0.05). At long-term follow-up, the rate of surgery at 1 year was 35%.
Several studies have investigated the prognostic factors of response to IFX in critically ill patients with/without steroid resistance. At the time of admission, high CRP levels, low albumin, positive pANCA antibodies, and severe endoscopic lesions were associated with a high likelihood of flare-ups or the need for surgery. Complete clinical response at weeks 10-14, endoscopic wound healing and serum IFX levels above 2.5 ug/ml at week 14 are good prognostic factors. for the possibility of no surgery and no recurrence. Low serum IFX concentrations at week 6 or high fecal IFX concentrations during the first days after initiation of treatment predict IFX nonresponse.
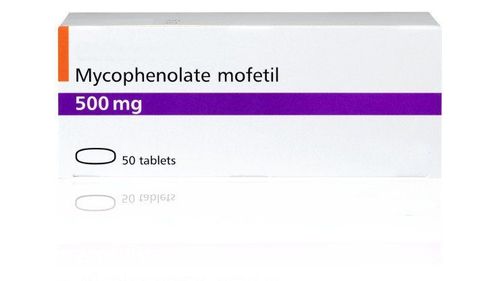
Head-to-head studies showed no difference between IFX and calcineulin inhibitors in the treatment of steroid-resistant patients on the criteria of quality of life, surgical rate, mortality, and infection. The choice of therapy still depends on the specific disease, Cyclosporine should not be used in patients with low cholesterol or 0.1g0e blood because of the increased risk of adverse effects on the nervous system. In patients with moderate disease activity resistant to oral steroids, intravenous steroids or anti-TNF preparations in combination with thiopurine or tacrolimus may be considered. . Steroid dependence For steroid-dependent patients, thieupurine, an anti-TNF preparations in combination with thiopurine or methotrexate can be used. In the event of failure, the second regimen may be switched to another biologic, vedolizumab, or surgery may be considered. The efficacy of anti-TNF agents such as infliximab, adalimumab, and golimumab in achieving and maintaining steroid-free remission has been demonstrated in multiple clinical trials with patients receiving initial corticosteroid therapy. The UC-SUCCESS trial demonstrated that the combination of IFX with azathioprine was more effective than IX alone. However, data on the combination of adalimumab or golimumab with immunomodulatory drugs are lacking. When there is no response to one anti-TNF drug, it is possible to switch to another drug. A meta-analysis showed that treatment response ranged from 23 to 92%, with remission rates ranging from 0 to 50% when switching from IFX to adalimumab. In Trial GEMINI 1, patients who had received steroid therapy but were dependent on or failed anti-TNF were randomized to receive Vedolizumab or placebo. At week 52, the steroid-free remission rate in the vedolizumab group was 38.5%, which was statistically significantly higher than in the placebo group (13.9%). In addition, no difference was observed between the achievement and maintenance rates of remission between the steroid-dependent group and the anti-TNF failure group. A multicenter study in patients with steroid-dependent bleeding ulcerative colitis demonstrated a statistically significant higher rate of remission at 16 weeks with methotrexate than in the placebo group (41). .7% versus 23.5%, p<0.05) and better symptom control.

2. Treatment of bleeding ulcerative colitis in immunomodulator-resistant patients
For patients with moderately advanced bleeding ulcerative colitis resistant to thiopurine, anti-TNF can be used in combination with thiopurine. If that fails, another anti-TNF product or vedolizumab can be switched. In case medical treatment does not bring clear clinical effect, surgery should be considered. Evaluation of resistance to immunomodulatory drugs should be based on endoscopic and histopathological results. It is also important to rule out possible causes of persistent symptoms such as CMV or C.difficile . In the absence of contraindications, anti-TNF agents should be selected. A Cochrane review enrolled patients with moderate to severe bleeding ulcerative colitis resistant to steroids and/or immunomodulators, using week 0.2 IV IFX and 6 would have a better rate of clinical symptom complete response week 8 than placebo. Several studies have also shown the efficacy of adalimumab and golimumab in patients who are intolerant to or have failed immunomodulatory therapy. ECCO still recommends that for this group of patients, it is still recommended to combine IX with thiopurine to prevent the creation of antibodies with biological products as well as to raise blood levels of IFX, thereby maintaining the effectiveness of treatment. No studies have demonstrated similar pharmacokinetic effects when adalimumab or golimumab are combined with immunomodulators.Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References 1. NagreF, Gionchetti PR, Eliakim R. et al. (2017). Third European Evidence-based consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and lleo- anal Pouch Disorders. J Crohn's Colitis, 11(6), 649-670. 2. De Dombal F.T. (1968), Ulcerative colitis: definition, historical background, aetiology, diagnosis, naturel history and local complications, Postgrad Med J,44(515), 684-692. 3. Crohn's B.B. (1962). An historic note on ulcerative colitis. Gastroenterology, 42, 366-367. 4. Lichtenstein G.R., btv. (2014), Medical Therapy of Ulcerative Colitis, Springer-Verlag, New York.