This is an automatically translated article.
Post by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General Hospital
Helicobacter pylori (H. pylori) is one of the factors implicated in the pathogenesis of various gastrointestinal diseases and may play a potential role in a number of extraintestinal diseases. Eradication therapy can improve the manifestations of Henoch-Schonlein purpura (HSP) and reduce the recurrence of Henoch-Schonlein purpura. Therefore, the results suggest that the detection of H. pylori infection by appropriate methods should be applied in children with Henoch-Schonlein purpura.
1. Overview
Current evidence indicates that local gastric mucosal injury and immunological events associated with H. pylori infection are associated with the development of Henoch-Schonlein purpura. Elevated serum IgA, cryoglobulin, C3 levels, autoimmunity, proinflammatory agents, and immune complex-inducing molecular mimics and antibodies that cross-react with H. pylori infection may play their part in the process. Henoch-Schonlein purpura. However, no investigation has confirmed a causal relationship between H. pylori infection and Henoch-Schonlein purpura, and the pathogenesis remains unclear. More bench studies and clinical studies are needed to establish a complex association between H. pylori and Henoch-Schonlein purpura.
2. Pathogenesis of Henoch-Schonlein purpura and H.pylori . infection
It is well known that the pathogenesis of Henoch-Schonlein purpura is still unclear. The clinical feature of Henoch-Schonlein purpura is the result of systemic leukemic vasculitis with immunoglobulin A (pIgA), activated complement (C3 or C5), and some fibrinogen/fibrin deposits in the vessel wall, no deposition of IgG or IgM. Immune complexes between these factors in the skin, intestines, kidneys, and other organs lead to purpura, intestinal manifestations, nephritis, and other relatively rare symptoms. Most investigators agree that IgA1 is important in the progression of Henoch-Schonlein purpura. Therefore, it can be speculated that any pathogen capable of initiating a type III allergic reaction with elevated serum IgA1 antibody levels and advanced systemic vasculitis may be indispensable. in Henoch-Schonlein purpura progression. H. pylori infection can also be diagnosed in patients with IgA nephropathy, which shares some similarities with Henoch-Schonlein purpura. High levels of anti-Hp serum IgA and glomerular pIgA disposition are two important factors among the features. H. pylori infection can induce a tilt toward serum IgA, C3 and cryoglobulin concentrations, which are thought to promote immune complex formation and increase the risk of Henoch-Schonlein purpura . A study in adult patients showed that, when compared with healthy controls, anti-Hp IgG levels during the acute phase of Henoch-Schonlein purpura and an anti-Hp IgA/IgG ratio during the remission phase. significantly higher. However, there is no solid evidence from prevention studies clarifying whether immune responses or abnormalities caused by H. pylori infection are associated with Henoch-Schonlein purpura or cause. the pathological process of the disease or not.
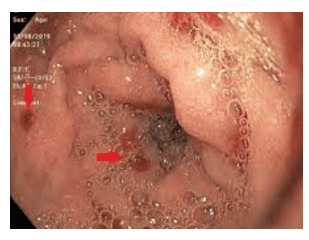
H. pylori infection leads to bacterial colonization of the gastric mucosa, resulting in direct damage to the physical barrier
Strong humoral and cellular immune responses can be induced. It is thought that such an immune response may coordinate the cross-talk between H. pylori infection and a number of extra-gastrointestinal diseases, including autoimmune processes, anti-inflammatory agents, and molecular mimics. immune complexes and cross-reactive antibodies. During this process, Ig A is secreted by the mucosa. Although this antibody is capable of inhibiting bacterial antigen reception, preventing H. pylori adhesion and migration, and neutralizing toxins, secretion is often over-activated. Prognosis of H. pylori infection is based on interactions between variant factors, such as virulence of the dominant strain, host characteristics, and environmental influences. The product of toxin gene A (vacA) and vacuole cagA are the major virulence factors of H. pylori. The vacA and cagA alleles, which encode the most important virulence proteins of H. pylori VacA and CagA, contribute to the isolation of bacterial strains in several Asian and Western countries for functional polymorphisms. Based on the high toxicity of Chinese H. pylori strains and the relatively low toxicity of strains in Western countries, we hypothesized that vacA or cagA might be involved in the progression of the disease. Henoch-Schonlein hemorrhage through a complex and unknown mechanism. Experimental studies on the relationship between H. pylori and atherosclerosis have shown that cagA antigen mimics vascular wall peptides, which also suggests that cagA antibodies will damage endothelial cells. Another study suggested that cagA increased IgA1 secretion in a dose/time-dependent manner. Furthermore, it was also shown that cagA can promote the sub-glycosylation of IgA1 in infected B cells. H. pylori also conducts a large secretion of inflammatory mediators, such as interleukin (IL)-6, IL- 12, IFN-γ, TNF-α, etc.
By their complex network of interactions, these cytokines participate directly or indirectly in the inflammatory response. The cellular immune response triggered by infection is another mechanism that may influence the course of Henoch-Schonlein purpura. It is reported that CD4+/Treg cell proliferation is stimulated by dendritic cells infected with H. pylori mediated by IL-1β, the secretion of which is stimulated by vacA and γ-glutamyl transpeptidase . However, no significant difference in Treg cell levels was identified between Henoch-Schonlein purpura patients and healthy controls. In contrast, Th17 cell activation was also reported to be a functional part of H. pylori-induced inflammation, and its concentration was shown to be higher in cases of Henoch-Schonlein purpura. These results suggest that further studies are needed on the cellular immune response underlying H. pylori infection. Molecular mimicry is another H. pylori approach to the induction of autoimmune diseases
For example, human Lewis determinants [Le(x) and/or Le(y)] and expression showed that H determinants could be detected in the majority of H. pylori isolates. While in some other strains the detected components changed to Le(a), Le(b) and sialyl-Le(x). All the determinants are in the O chain of the surface lipopolysaccharide. In preliminary studies, it has been shown that H. pylori is able to evade host responses and induce autoantibody responses to the Le antigen with the help of several initiating reactions. molecular imitation. Furthermore, one study hypothesized that autoantibodies against Le caused by H. pylori infection are associated with the progression of autoimmune disorders. However, there has been no clinical evidence to support this to date. The role of molecular mimics in immune disorders, such as Henoch-Schonlein purpura, requires further comprehensive analysis of T-cell and autoantibody functions. More functional and clinical studies may focus on the Le antigen and other components of the H. pylori surface lipopolysaccharide.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.