This is an automatically translated article.
Article translated by Dr. Nguyen Hong Thanh - Research Specialist, Vinmec High Technology Center
Recently approved liquid biopsy tests show great potential in helping to diagnose and plan effective treatment for cancer.
When a patient is likely to be diagnosed with cancer, doctors will recommend a local biopsy, which may include invasive procedures and, in some cases, the This is not possible. With tests that use liquid biopsy samples, doctors will have more options in making an early diagnosis of cancer with less invasive and potentially highly effective procedures. than.
Regarding this issue, recently, the US Food and Drug Administration (FDA) has approved two tests using liquid biopsy samples, one from Guardant Health and the other from Foundation Medicine. Both tests look for biomarkers of non-small cell lung cancer and solid tumors. Guardant Health's test, called Guardant360 CDx, is approved for all types of solid cancer by looking for mutations in the DNA of circulating cancer cells in the body. Foundation Medicine's test, called FoundationOne Liquid CDx, looks for mutations in about 300 genes in a single test.
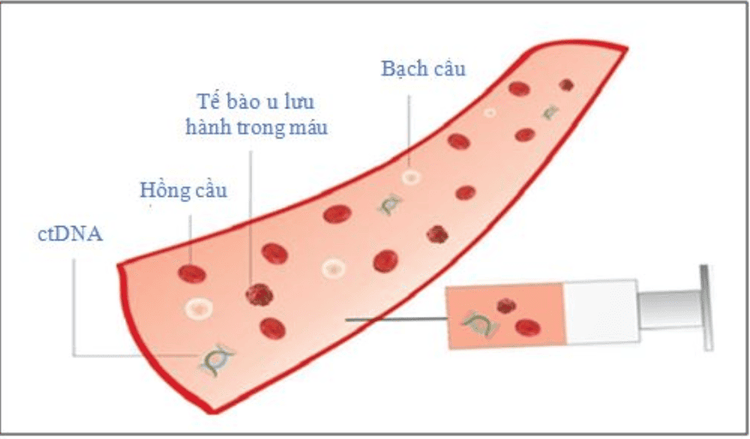
Sinh thiết lỏng có khả năng phát hiện sớm căn bệnh ung thư
The above tests can be used in the management of cancer treatment, by providing real-time assessments of disease status, especially if they are performed continuously.
According to FDA records, Guardant360 CDx is used in combination with the cancer drug osimertinib. Meanwhile, FoundationOne Liquid CDx is taken in combination with several targeted drugs including osimertinib, rucaparib and erlotinib.
Although no sample is taken directly from the tumor, liquid biopsy has the potential to provide useful information both early and in the treatment stage. Liquid biopsies can be performed more cheaply and in principle than tissue biopsies, and can therefore be used more widely and more frequently. From the liquid biopsy sample test results, doctors can monitor the progress of treatment and decide if a change in the treatment regimen is needed.
Matthew Freedman, researcher and oncologist at Dana-Farber Cancer Institute in Boston, USA, told USA Today that: liquid biopsies are gradually demonstrating their potential and growing position. mine.
A series of recent studies have shown that liquid biopsies are capable of detecting many types of cancer at different stages. One study has shown that liquid biopsies can detect more than 50 types of cancer and across all four stages of cancer.
Sinh thiết lỏng có thể phát hiện nhiều loại ung thư khác nhau
However, that does not mean that liquid biopsy is optimal or can replace conventional biopsy, because conventional tumor biopsy at the time of cancer diagnosis will provide important information. directly from the tumor. Therefore, it is unlikely that tumor biopsies will be 'replaced' by liquid biopsies," wrote Ryan B. Corcoran in the journal Nature Medicine.Instead, liquid biopsies could be used as a tool. The experimental evidence has shown that the method is reliable enough to be carried out in clinical trials, which, once implemented, will be an important step forward in the fight against cancer. Toni Choueiri told USA Today: "The best way to cure cancer is to stop it before it shows up, not with chemotherapy."
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
Article referenced source: thehill.com