This is an automatically translated article.
Posted by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General Hospital
Abbreviation
H. pylori-associated gastric cancer (HpPGC)
Stomach cancer not caused by H. pylori infection ( Stomach cancer not caused by H. pylori infection )
Helicobacter pylori ( H. pylori infection ) ) causes chronic inflammation, atrophy of the gastric mucosa and a high risk of developing gastric cancer. In recent years, awareness of H.pylori eradication treatment has increased in Japan. As H. pylori infection decreases, the incidence of gastric cancer arising from gastric mucosa that is not infected with H. pylori increases. The occurrence of gastric cancer arising in patients without H. pylori infection, although rarely reported, is a concern that needs to be addressed and should elucidate the clinical features of this disease. it.
1. How is H. pylori-free status determined?
Three criteria are used to determine whether a patient is infected with H. pylori: (1) No history of H. pylori eradication therapy, as determined by investigation of the profile. patient's medical records and conduct patient interviews; (2) There is no image of atrophic gastritis on endoscopy. As an additional finding, the authors consulted the endoscopic results of the Kyoto classification, including RAC (regular arrangement of collection sites). The endoscopic findings were subsequently confirmed by three skilled endoscopists (KH, CS and SM). And (3) check in laboratory tests including serology for H. pylori -IgG Antibody, Breath urease test (UBT), rapid urease test (RUT), and microscopy.
If the test results are negative for H. pylori after two or more tests, this is considered to be H. pylori free. Among patients with non-H. pylori gastric cancer, the presence or absence of pathological atrophy was assessed using the updated Sydney system on the mucosal background of ESD specimens. Tumors that met all three of the conditions described above were defined as gastric cancer not due to H. pylori infection.
2. Histological and endoscopic features of gastric cancer not caused by H. pylori infection
A study was performed in a total of 2462 patients with 3375 cases of early gastric cancer treated with endoscopic submucosal dissection who were enrolled in the author's study since May. May 2000 to September 2019. Of these, 30 lesions in 30 patients were diagnosed as gastric cancer not due to H. pylori infection (Stomach cancer not due to H. pylori infection).
According to the results of this study, histologically, gastric cancer lesions not caused by H. pylori infection were classified into basal adenocarcinoma (7 cases), differentiated adenocarcinoma good (8 cases), intestinal phenotypical carcinoma (7 cases) and simple signet ring cell carcinoma (8 cases). Histological and endoscopic findings of different types of lesions are explained below
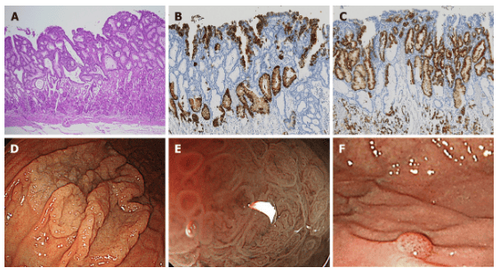
3. Basal adenocarcinoma (Figure 1):
Histopathological findings: HE staining revealed the presence of tumor cells mimicking basal glands at the mucosal base and tumor surface covered with noncancerous epithelium. Immunohistochemically, basal adenocarcinoma cells were positive for PG-I and MUC6, and part of the tumor expressed H+/K+-ATPase.
Six of the seven lesions showed submucosal invasion, while one of them showed SM2 invasion (distance from muscularis mucosae was 780 μm). None of the lesions showed lymphatic invasion. Endoscopy results: All seven lesions were located in the upper part of the stomach and were noted as small protrusions. With white light imaging, a yellow-white tumor covered with noncancerous epithelium was observed in submucosal tumors (SMTs).
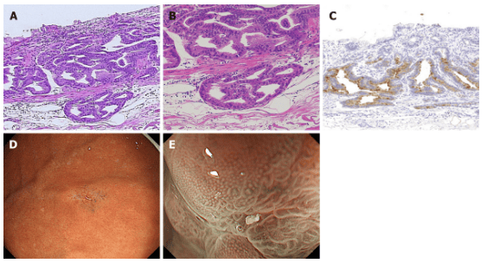
4. Well-differentiated alveolar adenocarcinoma type (Figure 2):
Histopathological findings: Most foci of gastric cancer are composed of dysplastic cylindrical cells with clear cytoplasm and have a villous or papillary structure mimicking alveolar epithelium. On the mucosal surface, enlarged glands that include non-tumor cells push onto the cancerous epithelium. The tumor was only superficial and the atrophy was recognized as a low-grade well-differentiated adenocarcinoma. Almost none of the lesions showed submucosal invasion, despite the large tumor size (mean diameter 37.3 ± 18.3 mm).
Nearly all alveolar gastric cancers are positive for MUC5AC, but negative for PG-I, MUC2, and CD10 are negative. MUC6 is positive for noncancerous glands extending mid to basal layer of mucosa. No lymphatic invasion was observed in any of the eight gastric cancers.
All lesions are located in the upper part of the stomach. Seven of the eight well-differentiated follicular adenocarcinomas were observed as highly spreading lesions with a white intestinal adenoma, and one lesion had a raspberry-like appearance. The ME-NBI showed a smooth papillary or villi-shaped mucosal pattern with structural irregular vessels in all lesions.
One of the well-differentiated alveolar adenocarcinomas is a small raspberry-shaped protrusion at the greater curvature of the upper stomach. Although this lesion resembles a hyperplastic polyp, the cell loss of the tumor suggests well-differentiated adenocarcinoma.
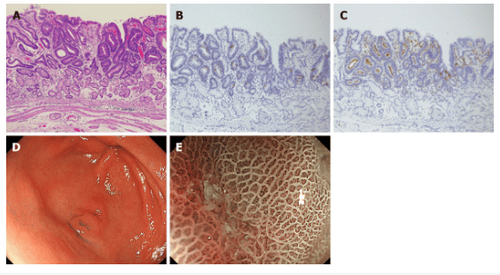
5. Intestinal phenotype carcinoma (Figure 3):
Histopathological findings: All tumors showed well-differentiated adenocarcinoma, characterized by a tubular structure lined by highly cylindrical cells with hyperchromic, pencillate, and pseudocyst nuclei. increase. Sharp Luminal contour with brush contour. Goblet cells were positive for MUC2 and the brush border of intestinal absorptive epithelial cells was positive for CD10. The superficial epithelium was completely positive for MUC5AC, and the deeper glands were positive for MUC6. These lesions are classified as gut-dominant mucin phenotypes. Endoscopic results: White light image shows mucosal mimic type 0-IIa + IIc lesions with red tint, approximately 5mm in size, and all lesions are found in the gastric wall. Unlike the common verrucosa, which often occurs in the antrum, it is characterized by only one or two bumps. An irregular microchip pattern and irregular microsurface pattern with demarcation line were observed in the concave region with ME-NBI.
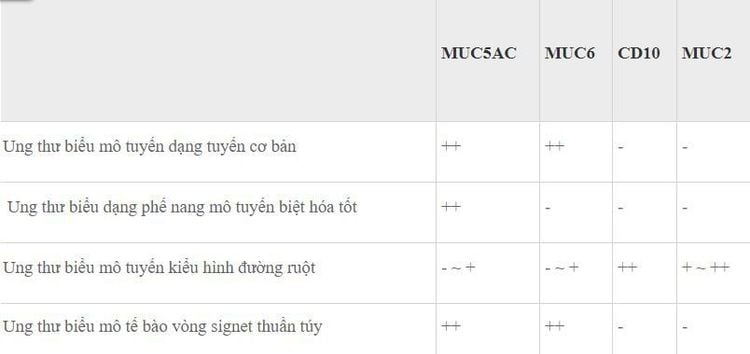
6. Mucin phenotype of non-H. pylori gastric cancer
All gastric cancer lesions not due to H. pylori infection were evaluated for their mucin phenotype (Table 3). Basal adenocarcinoma, well-differentiated adenocarcinoma, and pure ring cell carcinoma show a gastric phenotype or a dominant gastric phenotype, while squamous cell carcinoma gut phenotype exhibits a dominant gut phenotype.
Currently, Vinmec International General Hospital is a prestigious address trusted by many patients in performing diagnostic techniques for digestive diseases, diseases that cause chronic diarrhea, Crohn's disease, gastric mucosa Esophageal ectopic... Along with that, at Vinmec Hospital, screening for gastric cancer and gastric polyps is done through gastroscopy with Olympus CV 190 endoscope, with NBI (Narrow) function. Banding Imaging - endoscopy with narrow light frequency) results in clearer images of mucosal pathology than conventional endoscopy, detecting ulcerative colitis lesions, digestive cancer lesions Early stage...
Vinmec Hospital with modern facilities and equipment and a team of experienced experts, always dedicated to medical examination and treatment, customers can rest assured with internal services. gastroscopy and esophagoscopy at Vinmec International General Hospital.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
ReferencesUemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med . 2001;345:784-789. [PubMed] [DOI] Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum 1994; 61: 1-241. [PubMed] Parsonnet J, Vandersteen D, Goates J, Sibley RK, Pritikin J, Chang Y. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst . 1991;83:640-643. [PubMed] [DOI] Yamamoto Y, Fujisaki J, Omae M, Hirasawa T, Igarashi M. Helicobacter pylori-negative gastric cancer: characteristics and endoscopic findings. Digi Endosc. 2015;27:551-561. [PubMed] [DOI] Chiko Sato, Kingo Hirasawa et al., Clinicopathological features of early gastric cancers arising in Helicobacter pylori uninfected patients, World J Gastroenterol. May 28, 2020; 26(20): 2618-2631