This is an automatically translated article.
Post by Master, Doctor Mai Vien Phuong - Head of Department of Gastrointestinal Endoscopy - Department of Medical Examination & Internal Medicine - Vinmec Central Park International General Hospital
The available evidence demonstrates that liver damage in SARS-CoV-2 infection is the result of a multifactorial attack. The potential pathogenesis mechanisms may be broad-spectrum, ranging from direct cytotoxicity due to viral infection to indirect involvement of inflammatory cytokine storm, altered hypoxia due to respiratory failure, endocarditis and drug-induced liver injury (DILI).
1. Direct effect of viral infection on liver
Recently, it was determined by quantitative reverse transcriptase polymerase chain reaction that SARS-CoV-2 RNA is widely present in organs other than the respiratory tract, such as the liver. Although the exact cell site of replication has not been determined, because of the isolation of nucleic acids by homogenization of the whole tissue. Until recently, a typical hepatitis pattern has not been observed and the nutritional status of the liver, the direct cytotoxic effect of SARS-CoV-2 should be considered as the underlying mechanism of injury. liver associated with COVID-19.
A key determinant of viral organicity is the availability of viral receptors on the surface of host cells in specific tissues. Cell entry of SARS-CoV-2 is mediated by the virus mutant protein (S), which is cleaved by transmembrane serine protease 2 / transmembrane serine protease 4 and specifically interacts with the enzyme converting angiotensin 2 (ACE2) in the host (Figure 2).
According to the Human Protein Atlas, ACE2 is abundantly expressed in the lung (type II alveolar cells), intestine and gallbladder, but it appears to be almost absent in the liver. After in-depth investigation of ACE2 expression patterns, the sine endothelial cells appear to be negative for ACE2, but this protein is expressed in hepatic and portal vein endothelial cells.
The expression level of ACE2 in bile duct epithelium is comparable to that in alveolar epithelial cells, almost 20-fold higher than in hepatocytes. Notably, Letko et al revealed that compensatory differentiation and proliferation of bile duct cell-derived liver parenchyma cells lead to the upregulation of ACE2 expression in liver tissues, which is may be the underlying mechanism underlying COVID-19-induced liver damage.
MORE: Interaction of SARS-COV-2 with normal tissue and liver
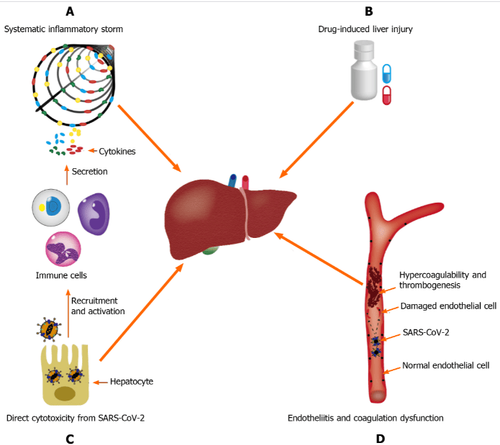
Interpretation of images:
A: Systemic inflammatory storm, produced by abnormal activation of the immune system; B: Drug-induced liver damage; C: Endocarditis and coagulation dysfunction; D: Direct cytotoxicity from severe acute respiratory syndrome coronavirus 2. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
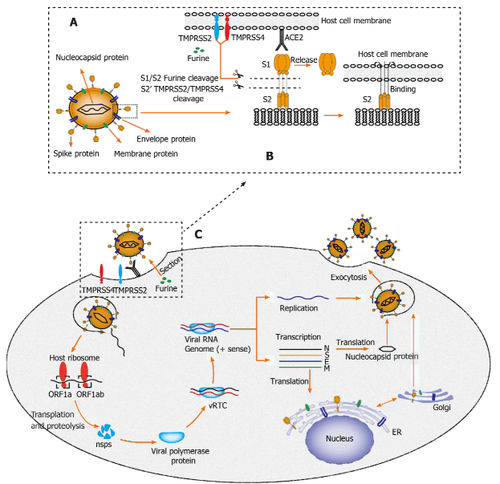
Interpretation of images
A: Structural sketch of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2);
B: Recognition and entry of SARS-CoV-2 into host cells. The transmembrane (S) spike glycoprotein on the surface of SARS-CoV-2 forms a homotrimer to recognize the human host angiotensin-converting enzyme 2 (ACE2) receptor. Protein S is specifically cleaved by two mucose-specific serine proteases [transmembrane recombinant serine protease 2 (TMPRSS2) and TMPRSS4] and furine. The S protein subunit (S1) is released and another subunit (S2) is exposed to and mediates viral entry into the host cell;
C: Life cycle of SARS-CoV-2 in host cells. First, the SARS-CoV-2 S protein binds to ACE2 to form the S protein-ACE2 complex, which directly mediates viral cell entry, and this process is facilitated. benefited by TMPRSS2, TMPRSS4 and furine. Second, viral RNA is released into the host cytoplasm. Open reading frame (ORF) 1a and ORF1ab are translated into large polyproteins by the host ribosome, which is further cleaved into 16 non-structural proteins (nsps). The viral protein polymerase is assembled by nsps and the viral replication/transcriptional complex (vRTC) is then formed mainly by protein polymerase and genomic RNA. Third, negative-sense viral RNA is synthesized and used as a template for progeny (+)-sensing viral genome replication and transcription to form different mRNAs. Nucleocapsid proteins are translated in the cytoplasm, while S proteins, membrane proteins (M) and envelope proteins (E) are translated in the endoplasmic reticulum and transported to the Golgi apparatus for further packaging. Finally, through the exocytosis of SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; M: Membrane; E: Envelopes; S: Spike; ER: Endoplasmic reticulum; vRTC: Viral Replication / Transcription Complex; ORF: Open reading frame; TMPRSS: Transmembrane serine protease; ACE2: Angiotensin-converting enzyme 2. Notably, typical coronavirus particles (characterized by the S structure) were identified in the cytoplasm of hepatocytes in a metastructural study by Wang et al. . In this study, lesions typical of viral infection, including conspicuous mitochondrial swelling, hypoglycaemia, and dilation of the endoplasmic reticulum, were also observed in hepatocytes infected with SARS-CoV-2, showed that liver failure could be directly caused by SARS- CoV-2. Interestingly, apoptosis in hepatocytes and some duplicated hepatocytes was also identified in this study.
In addition, autopsy results in patients with SARS, in the study by Guo et al., showed a large number of hepatocytes, central lobular necrosis, and obvious programmed cell death. clear. Similar histological findings were also observed in a study of liver biopsies from a SARS patient by Chau et al, suggesting that SARS-CoV may induce hepatocyte apoptosis, thereby lead to liver damage.
Furthermore, Tan et al demonstrated that overexpression of p7a, a protein specifically expressed in SARS-CoV-infected cells, can induce apoptosis in cell lines originating from various organs (including lungs, kidneys, and liver). This further confirms the possibility that SARS-CoV can directly attack liver tissues and cause liver damage.
2. The expression level of ACE2 on hepatocytes is regulated by many factors
It is noteworthy that the expression level of ACE2 on hepatocytes is regulated by many factors. Several experimental studies, in both rats and humans, have confirmed an increase in the expression of ACE2 in the liver under fibrotic/cirrhotic liver conditions. This may partly explain why patients with pre-existing chronic liver disease increase the probability of liver injury in COVID-19 patients.
Hypoxia, a typical feature in severe COVID-19 cases, has been shown to be a key regulator of ACE2 expression in hepatocytes. This may explain why dissemination of SARS-CoV-2 outside the lungs is mainly observed in patients with acute respiratory distress syndrome and other hypoxic conditions. Notably, the affinity of the S protein in SARS-CoV-2 for its receptor can be increased when it is protein-activated by trypsin, a protein commonly expressed in liver epithelial cells.
A clinical drug trial by Fantini et al. indicates that ganglioside (GM1) may be another target affecting S-ACE2 protein interactions, using a combination of structural and molecular modeling approaches death. In the near future, new molecular and therapeutic insights related to the S protein-ACE2 interactor are expected to be explored with the advancement of research.
Follow Vinmec International General Hospital website to get more health, nutrition and beauty information to protect the health of yourself and your loved ones in your family.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References Cai Y, Ye LP, Song YQ, Mao XL, Wang L, Jiang YZ, Que WT, Li SW. Liver injury in COVID-19: Detection, pathogenesis, and treatment. World J Gastroenterol 2021; 27(22): 3022-3036 [DOI: 10.3748/wjg.v27.i22.3022]