This is an automatically translated article.
Zopylas contains zoledronic acid as the main ingredient. Effective in the treatment of malignant tumors causing hypercalcemia, bone metastases due to cancer or malignancies, bone metastases due to cancer. To ensure the effectiveness of using Zopylas, users need to follow the instructions of their doctors, and refer to more information about the uses of Zopylas in the following article.1. What are the uses of Zopylas?
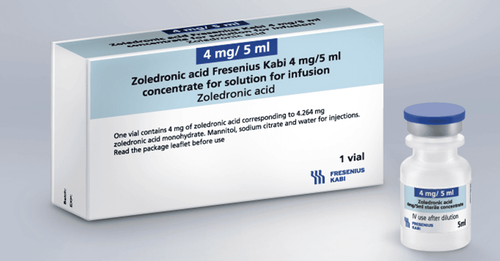
1.1. What is Zopylas? Zopylas belongs to the group of drugs that fight cancer and affect the immune system, with registration number VD-29986-18, manufactured by Pymepharco Joint Stock Company - Vietnam. Zopylas is prepared in the form of a concentrated solution for injection with a capacity of 5ml, a box of 1 vial. Zopylas is recommended for use in adults. 1.2. What does Zopylas do? Zoledronic acid has a major effect on bone. Up to now, this is believed to be the most potent inhibitor of osteoclast-induced bone resorption without affecting mineralization, bone formation or bone mechanical properties.
Zopylas is prescribed by doctors in cases involving bone:
Treatment of hypercalcemia caused by malignancies. Cancer or bone damage caused by multiple myeloma leads to bone metastases. Paget's disease of bone. Prevention and treatment of osteoporosis (for postmenopausal women, elderly men, osteoporosis caused by corticosteroid use). Contraindications:
Patients who are allergic to the main ingredient Zoledronic Acid or any of its ingredients. Patients with hypocalcemia. Patients with acute and chronic renal failure. Subjects are pregnant and lactating women Do not take Zopylas with other bisphosphonates.
2. Usage of Zopylas
2.1. How to take Zopylas Zopylas is administered intravenously. Dilute 5 ml of a solution containing 4 mg of zoledronic acid with 100 ml of a 5% glucose solution or 0.9% sodium chloride. Administer intravenous infusion over at least 15 minutes. Dosage: Withdraw an appropriate volume to obtain the corresponding amount of drug from Zopylas 4mg/5ml solution as follows: 4.4ml corresponds to a dose of 3.5mg. 4.1 ml corresponding to the dose of 3.3 mg or 3.8 ml corresponding to the dose of 3 mg. The amount of solution withdrawn was diluted in 100 ml of 5% glucose solution or 0.9% sodium chloride solution.
2.2. Dosage of the drug Zopylas Treatment of hypercalcemia caused by malignancies:
Use a single dose of 4mg (equivalent to 5ml), repeated after at least 1 week (if necessary). The maximum dose is 4mg/time because an overdose increases the risk of adverse effects on the kidneys. Bone metastases due to cancer:
Use a single dose of 4mg, IV every 3 to 4 weeks. Bone marrow pain:
Single dose 4mg, every 3 to 4 weeks. Simultaneous supplementation of calcium 500mg (oral) and polyvitamin containing 400 units of vitamin D. Paget's disease of bone:
Use a single dose of 4mg. To reduce the risk of hypocalcemia due to zoledronic acid, it is necessary to supplement with 1.5g of calcium per day (equivalent to a dose of 750mg twice a day or 500mg three times a day) and 800 units of vitamin D per day. Especially during the first 2 weeks after treatment with Zopylas. Treatment of osteoporosis (postmenopausal or corticosteroid-induced):
A single dose of 4mg once a year. Prophylaxis of postmenopausal osteoporosis
Single dose 4mg, intravenous infusion every 2 years. Dose adjustment in patients with renal impairment is based on creatinine levels:
Creatinine levels above 60 ml/min: 4 mg (no dose adjustment required). Creatinine concentration from 50 - 60 ml/min: 3.5 mg. Creatinine concentration from 40 to 49 ml/min: 3.3 mg. Creatinine concentration from 30 to 39 ml/min: 3 mg. Creatinine levels below 30 ml/min: Not recommended. Treatment when missed dose:
Zopylas is administered by medical staff at medical facilities, so it will limit the missed dose. If a missed dose occurs, initiate the infusion as soon as possible; if it is almost time for the next dose, skip the missed dose and take a new dose as directed. Never inject a double dose and do not miss 2 doses in a row. Overdose management:
Symptoms of overdose are usually hypocalcemia (muscle spasms, tingling sensations, anxiety, depression, difficulty swallowing, difficulty speaking, fatigue, and vision problems... ) If any serious side effects appear during the use of Zopylas, it is necessary to notify the doctor immediately for timely treatment. You may need intravenous calcium. Also monitor renal function because zoledronic acid can cause renal failure.
3. Note when using Zopylas
Before and after treatment with Zopylas, the body must be made sure to have enough water because a lack of water in the body increases the risk of kidney damage. But avoid drinking too much water for people at risk of heart failure. Do not use Zopylas after the expiry date, feel cloudy or the vial seal is open. The active ingredient zoledronic acid in Zopylas affects calcium more than other bisphosphonates, so it also increases the risk of causing severe hypocalcemia leading to paresthesia. Therefore, it is necessary to treat hypocalcaemia and correct factors related to bone or mineral metabolism (including malabsorption, hypoparathyroidism, small bowel resection, thyroid surgery, surgery, etc.) parathyroid surgery...) before using Zopylas in people with hypercalcemia due to malignancy. Careful monitoring of metabolic parameters associated with hypercalcemia is required. If hypocalcaemia, hypophosphataemia, or hypomagnesemia are encountered, additional treatment will be required for a short time. Usually, patients with untreated hypercalcaemia are at higher risk for renal dysfunction, so renal function should be closely monitored. Side effects of Zopylas medicine include headache, dizziness and blurred vision, so it will affect the ability to drive and use machines. Use caution in this case. Nursing mothers: Zopylas is stored in the bones for a long time, so this object should not be used. The study of using Zopylas for pregnant women has not yet, and in animals, it has been found that Zopylas remains in the bones of the fetus more than in the bones of the mother even after the end of treatment, so Zopylas should not be used. for this object.
4. Side effects of the drug Zopylas
At therapeutic doses, Zopylas is well tolerated. However, during the use of Zopylas, patients may still experience side effects such as:
Common:
Anemia and hypophosphataemia. Flu-like syndrome (including myalgia, bone pain, arthralgia, fever, and muscle stiffness). Dizziness, headache and conjunctivitis. Gastrointestinal disturbances, renal failure (rarely acute renal failure) and atrial fibrillation. Uncommon:
Taste disturbance, anorexia, decreased blood pressure and dyspnea. Changes in sensation, coughing, muscle tremors, and anxiety. Stomatitis, dry mouth, chest pain and hypertension. Sleep disturbance, weight gain, blurred vision, hematuria and proteinuria. Erythema, itching, sweating and cramps. Hypersensitivity reactions (such as angioedema), peripheral edema, asthenia, reactions at the injection site. Leukopenia, thrombocytopenia, hypokalemia and hypomagnesaemia. Rare:
Increased K+, increased Na+ blood, bradycardia, confusion, choroiditis and scleritis. Pancytopenia, osteonecrosis of the jaw, atypical femoral fractures. If you experience these symptoms, the patient should stop using Zopylas and notify the doctor for appropriate treatment.
5. Zopylas . drug interactions
Co-administration of Zopylas with loop diuretics, aminoglycoside antibiotics will cause synergistic effects to increase the risk of hypocalcemia. Co-administration of Zopylas with nephrotoxic drugs, thalidomide, non-steroidal anti-inflammatory drugs increases the risk of adverse effects on the kidneys. Absolutely do not mix Zopylas with calcium solutions, infusion solutions containing divalent cations such as Ringer lactate solution for infusion. To avoid interactions, before being prescribed Zopylas, the patient should inform the doctor about the drugs they are using, including functional foods. The doctor will base on that to prescribe the appropriate Zopylas.
6. How to store Zopylas
The shelf life of Zopylas is 36 months from the date of manufacture. Zopylas medicine is stored at room temperature below 30 degrees Celsius, in a cool and dry place, avoiding direct sunlight. Because high temperatures can damage, damage or change the ingredients in the medicine. Do not leave Zopylas in places with high humidity such as bathrooms, refrigerators. Store Zopylas pills out of reach of children. Above is all information about Zopylas, patients need to carefully read the instructions for use, consult a doctor / pharmacist before using. Note, Zopylas is a prescription drug, patients do not arbitrarily use to ensure safety for health.