This is an automatically translated article.
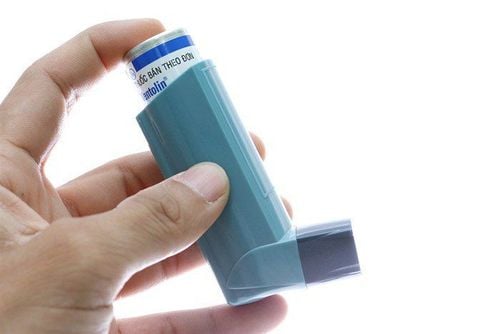
Symbicort 60 has the main ingredients Budesonide, Formoterol fumarate. Symbicort is indicated in the treatment of asthma and chronic obstructive pulmonary disease. Learn how to use Symbicort in the article below.
1. What is Symbicort?
Symbicort 60 has the main ingredient budesonide, formoterol fumarate.
Budesonide is an inhaled glucocorticosteroid that exerts dose-dependent anti-inflammatory effects in the respiratory tract, reducing symptoms and reducing asthma exacerbations. Formoterol is a selective beta-2 agonist that, when inhaled, causes rapid and prolonged relaxation of bronchial smooth muscle in patients with reversible airway obstruction.
2. What does symbicort do?
Symbicort 60 is indicated for use in the following cases:
Asthma : Adults and adolescents (12 years of age and older) in the routine treatment of asthma (asthma) when combination therapy with corticosteroid form is required. Inhaled and long-acting inhaled beta-2 agonists: Patients not well controlled with inhaled corticosteroids and short-acting inhaled beta-2 agonists used as needed. The patient was well controlled with inhaled corticosteroids and long-acting beta-2 agonists in individual inhalers. Chronic Obstructive Pulmonary Disease: Adults 18 years of age and older in the symptomatic treatment of patients with chronic obstructive pulmonary disease with forced expiratory volume in first second (FEV1) < 70% of the estimated normal (after bronchodilator) and a history of exacerbations despite regular bronchodilator therapy.
3. How to take Symbicort
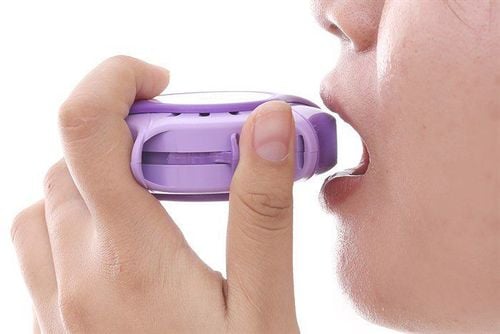
How to use Symbicort as follows:
The patient inhales strongly and deeply through the mouthpiece. Do not exhale through the mouthpiece. Close the lid after use. Gargle after inhalation.
4. Undesirable effects of the drug Symbicort
Some common side effects of Symbicort drugs such as: nervousness, headache, tremor, mild irritation in the throat, cough, hoarseness, oropharyngeal candidiasis, pneumonia (in COPD patients).5. Contraindications of the drug Symbicort
Symbicort is contraindicated in cases of hypersensitivity (allergy) to Budesonide, Formoterol or Lactose (which contains small amounts of milk proteins).
6. Be careful when using Symbicort
Gradually reduce the dose of Symbicort when stopping treatment and should not stop suddenly. If the patient feels that the treatment is not effective or the dose is exceeded, the doctor must be monitored. Patients always carry an asthma reliever such as Symbicort or a separate rapid-acting bronchodilator. Remember to take the maintenance dose of Symbicort as prescribed even in the absence of symptoms. Once symptoms are under control, a gradual dose reduction should be considered. Symbicort treatment should not be initiated if the patient is in the midst of an asthma exacerbation, or if the condition worsens or is acute. Adverse events related to asthma and asthma exacerbations may occur during treatment with Symbicort. Patients can be continued on Symbicort but should consult their doctor if their asthma symptoms are not controlled or worsen. Systemic effects may occur especially when high doses are used for a long time. Possible effects include Cushing's syndrome, adrenal suppression, growth retardation in children and adolescents, glaucoma, decreased bone mineral density, and cataracts. To reduce the risk of oropharyngeal candidiasis, patients should rinse their mouth with water and then spit it out after each inhalation of the maintenance dose. Use with caution in patients with thyrotoxicosis, pheochromocytoma, untreated hypokalemia, diabetes mellitus, severe hypertension, hypertrophic obstructive cardiomyopathy, idiopathic subvalvular aortic stenosis, aneurysm or other serious cardiovascular disorders such as ischemic heart disease, tachycardia, severe heart failure. Use caution in patients with prolonged QT interval. Use caution when using the drug in patients with active or latent pulmonary tuberculosis, fungal infections, respiratory viruses. Children: It is recommended that the height of children undergoing long-term therapy be monitored. If developmental delay is present, treatment should be re-evaluated to reduce the dose of inhaled corticosteroid to the lowest dose that still controls asthma. Ability to drive and use machines: The drug has no or negligible influence on the ability to drive and use machines. Pregnancy: There are no data on the use of Symbicort in pregnant women. Lactation: Budesonide is excreted in breast milk. However, at therapeutic doses, no effects have been observed in breastfed infants. It is not known whether formoterol is excreted in human milk. Consider Symbicort for nursing women if the potential benefit to the mother outweighs the possible risks to the infant. Above is information about uses, dosage and precautions when using Symbicort. To ensure safety for health and maximize the effectiveness of treatment, patients need to use Symbicort medicine exactly as directed by the doctor.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.