This is an automatically translated article.
Post by Master, Doctor Mai Vien Phuong - Head of Department of Gastrointestinal Endoscopy - Department of Medical Examination & Internal Medicine - Vinmec Central Park International General Hospital
Accumulating clinical data shows that liver damage is related to the severity of COVID-19 and is also a major cause of death from COVID-19, especially when liver failure is present. Therefore, early detection, effective treatment and elucidation of the pathogenesis of liver injury are urgent for COVID-19 patients.
1. Overview
Coronavirus disease 2019 (COVID-19) caused by coronavirus 2 (SARS-CoV-2), which causes severe acute respiratory syndrome, first broke out in Wuhan, China in December 2019. The disease has returned a major threat to public health worldwide. As of August 4, 2020, more than 1000000 deaths from COVID-19 have been confirmed. Although the lungs are the main organ damaged in COVID-19, approximately 60% of patients have been reported to develop varying degrees of liver damage in previous studies.
Accumulating clinical data shows that liver damage is related to the severity of COVID-19 and is also a major cause of death from COVID-19, especially when liver failure is present. Therefore, early detection, effective treatment and elucidation of the pathogenesis of liver injury are urgent for COVID-19 patients. In this review, the authors summarize the features of COVID-19-associated liver injury from multiple perspectives, including clinical features (presentation, laboratory examination, liver biopsy, etc.). ).
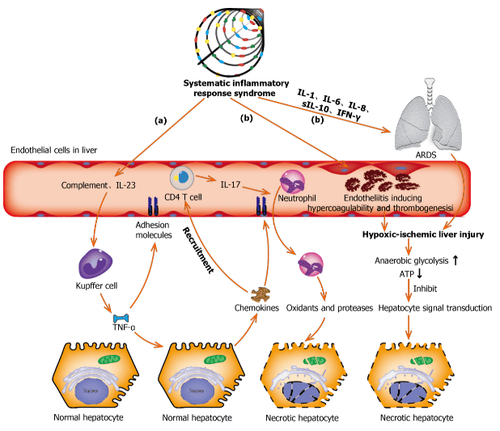
Interpretation of the image
(a): Complement and interleukin-23 are released into the bloodstream during systemic inflammation, which then activates Kupffer cells and induces tumor necrosis factor α (TNF-α) ) of them. As an anti-inflammatory cytokine, TNF-α exacerbates inflammatory responses by upregulating the expression of endothelial cell adhesion molecules and causing hepatocytes to secrete chemokines. Under the induction of chemokines, CD4 T cells and neutrophils are rapidly recruited to the liver, where CD4 T cells support mucosal molecules that promote neutrophils into the liver parenchyma. Finally, neutrophils directly damage hepatocytes by releasing oxidants and proteases, leading to necrotic cell death;
(b): Acute respiratory distress syndrome and endocarditis are the two main causes of hypoxic-ischemic liver injury during the systemic inflammatory response syndrome phase. Increased anaerobic glycolysis leads to decreased ATP production, which eventually leads to hepatocyte death due to inhibition of hepatocyte signal transduction. ARDS: Acute respiratory distress syndrome; TNF-α: Tumor necrosis factor α; IL: Interleukin; IFN-γ: Interferon-γ.
2. Clinical features of liver injury in patients with covid-19
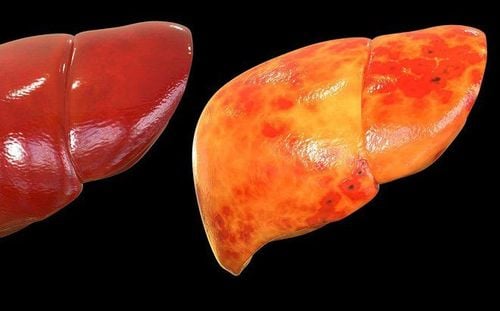
In patients with COVID-19, commonly used indicators to assess liver function decline are liver transaminases, bilirubin, and albumin levels. Abnormal levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin were reported as 11%-56.3%, 15.0%-86.8%, and 2.7%-30, respectively. .6% of patients had COVID-19. While 2%-11% of such patients had pre-existing liver disease. In a recent study involving 228 patients with COVID-19, who did not have chronic liver disease (CLD), abnormal liver function was observed in 129 (56.3%) patients, including: including elevated levels of ALT [84 (36.8%)], AST [58 (25.4%)], total bilirubin [59 (25.9%)], and gamma-glutamyl transferase [67 (29.5) %)]. In a study of 99 patients, Chen et al reported elevated ALT and AST levels in 28 (28%) and 35 (35%) patients and hypoalbuminemia and hyperbilirubinemia in 97 (98%) and 18 patients. (18%) of the respective patients.
Additionally, some studies show that liver damage is more common in severe cases of COVID-19 than in mild cases. In a large sample multicenter study, abnormally elevated ALT levels were observed in 28.1% of critically ill and 19.8% of non-severely ill patients and AST in 18.2% of patients. not seriously ill and 39.4% of the critically ill. Huang et al also showed that patients admitted to the intensive care unit (ICU) not only had higher plasma levels of inflammatory markers [interleukin (IL)-2, IL-7, IL-10, weak tumor necrosis factor (TNF)-α, etc. . ], but also had abnormally high AST levels [8 (62%) of 13 patients] compared with non-ICU patients [7 (25%) of 28 patients]. A recent descriptive study confirmed that ALT levels (35 vs 23, normal range 9-50 U/L, P = 0.007) and AST (52 vs 29, normal range 5-21 U) /L, P < 0.001) was significantly higher in ICU patients.
In a Japanese cohort study related to COVID-19, patients were classified into mild, moderate and severe groups, respectively, based on gastrointestinal symptoms and severity of pneumonia. The same peak levels of AST (28 vs 48 vs 109, P < 0.001) and ALT (33 vs 47.5 vs 106, P = 0.0114) were significantly stratified by these criteria.
3. Characteristic clinical manifestations of liver damage
In addition to the liver enzyme tests mentioned above, there are also typical clinical manifestations of liver damage. In China, it was reported that some patients recovering from severe COVID-19 showed darkening and melasma during their recovery. Damage to multiple organs, especially liver damage, is the main cause of skin darkening and hyperpigmentation. Abnormal liver function can affect pigmentation in three ways:
(1) Impaired liver function leads to impaired function of the adrenal cortex. When the liver is unable to metabolize the melanin-stimulating hormone secreted by the anterior pituitary gland, the secretion of melanin is increased; (2) Abnormal liver function interferes with the inactivation of estrogen, resulting in increased levels of it. An increase in estrogen levels in the body reduces the inhibition of tyrosinase by thiamine, thereby increasing the conversion of tyrosin to melanin; (3) Liver damage increases the iron content in the blood. Iron is provided to the facial skin, causing the facial skin to darken.

4. The role of liver biopsy
Liver biopsy is also important in diagnosing the etiology of liver damage in patients with covid-19, especially in cases where liver damage predominates clinically or when other causes of damage need to be ruled out. other substitutes. Currently, most information on histological changes in the liver of patients with COVID-19 comes from autopsies. The case series by Bradley et al, based on the ultrastructural and histopathological findings of samples from 14 fatal COVID-19 infections in Washington State, showed central necrosis, consistent with hypoperfusion lesions, in four patients. Accordingly, viral RNA was also detected in the livers of these patients. However, autopsies have some limitations. Death can occur long after acute liver injury is noted. Thereafter, histological changes may have been eliminated or obscured, and the viral load would decrease over time.
Fiel et al presented their findings from two liver biopsies performed on patients infected with SARS-CoV-2. Both patients had severe liver failure in the absence of obvious other organ involvement. Detailed in situ histological analysis and electron microscopy revealed that apoptosis, multiple mitosis, mixed inflammatory infiltrate in the portal region, severe bile duct injury, clear viral particles and viral RNA in Hepatocytes are typical.
These findings suggest liver involvement in SARS-CoV-2 infections. Another case report by Melquist et al showed similar findings in a patient infected with SARS-CoV-2, presenting as acute hepatitis without any respiratory symptoms, rapidly. progression to fulminant liver failure. Acute hepatitis (panacinar hepatitis, zone 3 necrosis, and focal haematopoiesis) with viral-like changes was identified at the time of liver biopsy.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References Cai Y, Ye LP, Song YQ, Mao XL, Wang L, Jiang YZ, Que WT, Li SW. Liver injury in COVID-19: Detection, pathogenesis, and treatment. World J Gastroenterol 2021; 27(22): 3022-3036 [DOI: 10.3748/wjg.v27.i22.3022]