This is an automatically translated article.
Post by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General Hospital
RON receptor tyrosine kinase (RTK; also known as MST1R) was first identified in 1993 in a cDNA library from human epithelial cells. RON belongs to the c-MET family of proto-oncogenes. This RTK family has only two members, RON and Met, with only 34% similarity overall; however, the tyrosine kinase regions of the receptors were quite similar at 80% similarity.
In 1994, a mouse cDNA was cloned encoding a homolog of RON, called the stem cell-derived tyrosine kinase receptor. RON is located on human chromosome 3p21, and this gene shows a high degree of conservation in various species, including xenopus, zebrafish, chickens, cats, humans, and mice. The RON receptor is initially synthesized as a biologically inactive single-chain precursor (pro-RON), which is then cleaved into a 145 kDa β chain and a 35 kDa extracellular alpha chain, which are linked by linkage binds disulfide, forming a mature receptor.
1. RON receptor and macrophage-stimulating protein
In 1994, the physiological ligand of RON was identified as a macrophage-stimulating protein (MSP) [also known as hepatocyte growth factor-like protein (HGF)], establishing a macrophage-stimulating protein signaling system. -RON phagocytosis. Macrophage-stimulating protein is a member of the plasminogen-associated kringle protein family. The human macrophage-stimulating protein gene is also located on chromosome 3p21 and is evolutionarily conserved in different species, similar to RON. The main source of macrophage-stimulating protein is hepatocytes, and the macrophage-stimulating protein circulates in the blood as the macrophage-stimulating pro-protein, which is a biologically inactive single-chain precursor. Following the subsequent proteolytic transformation, the active adult macrophage-stimulating protein consists of a disulfide-binding alpha subunit and a β chain. The high-affinity binding site of the RON receptor is located in the β chain, and RON activity is regulated by the alpha chain. Binding of macrophage-stimulating protein to RON induces RON isomerization, which activates multiple downstream signaling pathways, leading to RON-mediated cell growth, survival, and invasion
2. Unusual RON activation in different tumor types
In the last two decades, many studies have focused on the tumorigenic and therapeutic role of RON signaling. Although there are few studies regarding pathologically relevant changes in macrophage-stimulating protein expression, many studies regarding aberrant RON activation in different tumor types have been reported. published, including overexpression of the RON protein, oncogenic variant generation, and ongoing activation of downstream signaling pathways. In addition, tumor progression and malignancy are related to functional crosstalk between signaling proteins and RON. In clinical application, increased RON expression can be used to prognosticate patient survival and disease progression. Hepatobiliary and pancreatic cancers (HBP) have poor outcomes, with high cancer mortality because of high rates of recurrence, metastasis, and invasion, and are insensitive to chemotherapy. Total surgical resection remains the most effective treatment for HBP cancer. Among these cancers, the 5-year survival rate for liver cancer is about 30%, while for cholangiocarcinoma and pancreatic cancer, the rates are less than 30% and less than 10%, respectively. The high mortality rate of pancreatic cancer is due to the lack of early diagnosis and effective treatment. In pancreatic cancer, most cases are diagnosed when the disease is advanced, and only 20% or less of patients with locally curable tumors can be surgically removed. Therefore, the identification of a new potential therapeutic strategy is necessary. A growing body of evidence suggests a close relationship between HBP cancer and RON dysregulation. Therefore, the present review mainly focuses on the role of RON in the pathogenesis of cancer, especially HBP cancer. Furthermore, we summarize the latest advances in the development of RON-targeting strategies as a potential HBP cancer therapy.3. Role of RON and C-met in cancer pathogenesis
RON and c-MET, both members of the semaphorin family of transmembrane receptor tyrosine kinases, share similar structural and biochemical properties. The proteins exist as heteromers consisting of extracellular and transmembrane chains linked together by disulfide bonds. The extracellular sequences RON and c-MET have very similar functional domains, including SEMA, which regulates phosphorylation, receptor dimerization, and ligand binding. RON and c-MET are activated by their respective ligands: macrophage-stimulating protein for RON and hepatocyte growth factor-like protein for c-MET. c-MET and hepatocyte growth factor-like protein are expressed in a variety of cells and tissues. In contrast, RON is tightly restricted to epithelial stem cells, while hepatocytes are the major source of its ligand, the macrophage-stimulating protein. The ligand-independent or dependent activation of RON and c-MET induces substrate entry, cell migration, and cell proliferation, all of which are important for embryogenesis, healing, and embryogenesis. wound and tumor formation. There is increasing evidence identifying the role of RON and c-Met in the pathogenesis of cancer.
For example, c-MET and RON overexpression has been observed in a variety of primary and metastatic tumors, leading to aberrant downstream signaling activation, which contributes to growth and progression development of cancer. Furthermore, clinical studies have confirmed that increased expression of RON and c-MET is a prognostic factor for predicting survival and disease progression in some cancer patients. Furthermore, the activation of RON and c-MET promotes the malignant phenotype of cancer cells. Increased RON and c-MET expression promotes tumor cells to undergo epithelial-to-mesenchymal transition (EMT), which is characterized by loss of epithelial properties and increased mesenchymal features. . The increased c-MET and RON expression also contributed to the acquired chemoresistance. Given the above-mentioned role of increasing c-MET and RON expression in cancer pathogenesis, targeting RON and c-MET represents a promising cancer therapeutic strategy.
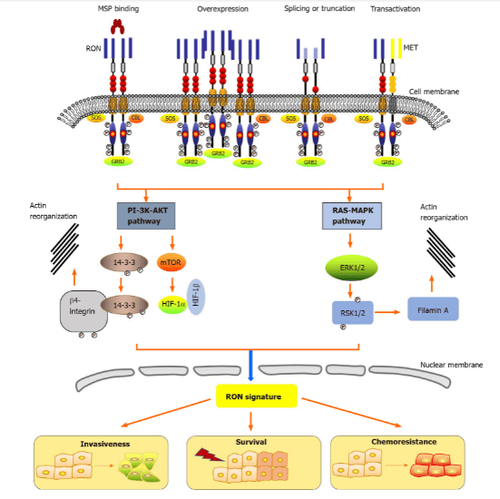
4. Activation mechanisms and signaling pathways of ron
Epithelial cells in the skin, adrenal glands, bones, brain, kidneys, intestines, lungs, and liver express low levels of RON. RON activity plays an important role in epithelial cell motility, enhancement of adhesion, sperm motility in the epididymis and embryonic development, as well as regulation of spermatogenesis. inflammatory reactions. Under physiological conditions, the primary cause of RON activation is stimulation of its ligand, the macrophage-stimulating protein.Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References:Chen SL, Wang GP, Shi DR, Yao SH, Chen KD, Yao HP. RON in hepatobiliary and pancreatic cancers: Pathogenesis and potential therapeutic targets. World J Gastroenterol 2021; 27(20): 2507-2520 [DOI: 10.3748/wjg.v27.i20.2507]