This is an automatically translated article.
Article by Dr. Nguyen Khanh Hoa - Vinmec Stem Cell Research Institute and Gene Technology.A novel alternative to bariatric surgery is pharmacotherapy based on intestinal peptides (Figure 2). The gut is a source of many factors, mainly peptides, that regulate intestinal motility and nutrient absorption. But intestinal peptides also function to promote other organs for the availability of nutrients and to initiate counter-regulatory responses such as satiety.
Here, we highlight some of the major intestinal peptides in terms of secretory sites, effector sites and organs, and functions, with emphasis on molecules with therapeutic potential discovered in studies. clinical study.
Some of these peptides, particularly GLP-1, but also others glucagon-related peptides, such as glucose-dependent insulinotropic glucosepolypeptide (GIP) and oxyntomodulin (OXM) as well as the tyrosine tyrosine peptide (PYY), have become important therapy for obesity and type 2 diabetes. The observed effects place them among the most intriguing new therapeutic approaches in these areas. How these gut-derived hormones work and how they can work together potentially mimicking the effects of bariatric surgery is an exciting area of research that could potentially lead to Complementary therapies for diabetes and obesity.
T2DM.
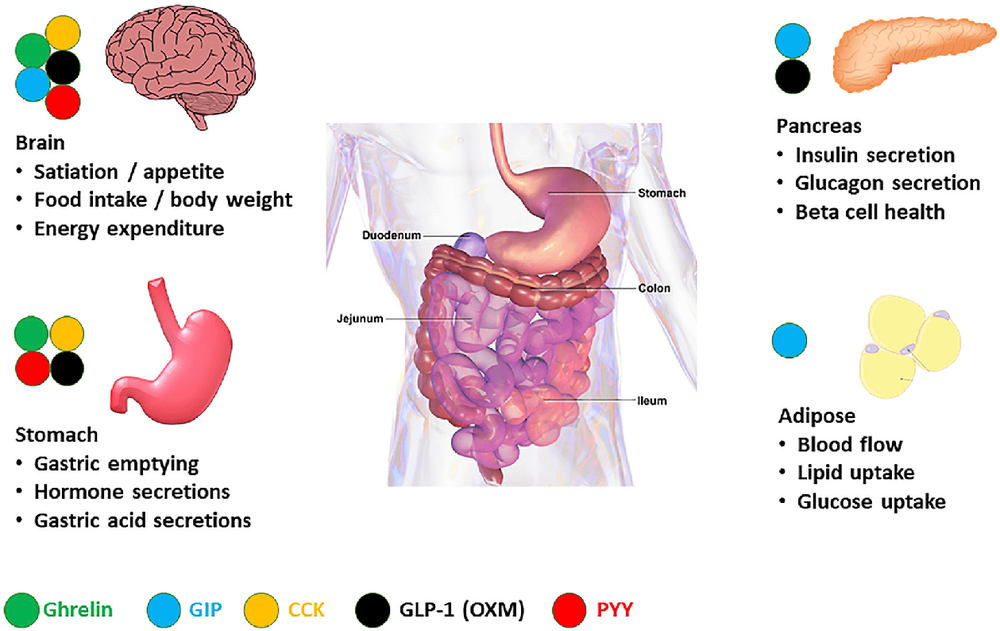
Hình 2. Hormone ruột chính điều chỉnh chức năng trao đổi chất
GLP-1 . receptor agonist
GLP-1, by itself, is useful for characterizing the physiology and pharmacology of incretin but is not suitable for therapeutic use due to its very short half-life. Exendin-4, like the GLP-1 Peptide, isolated from the saliva of the Gila monster, is more stable, allowing the development of therapeutics and exenatide, a synthetic version of exendin-4 administered as a double injection. per day, is the first GLP-1-based therapy for the treatment of T2DM to be commercialized. Subsequently, an appropriate long-term GLP-1 receptor agonist for once-daily or once-weekly administration, including an exenatide regimen, was approved for clinical use.Several important patterns have been discovered through clinical studies of GLP-1 receptor agonists. All molecules approved to date show potent hypoglycemic effects, partly because they act directly on beta cells to stimulate glucose-dependent insulin secretion, but also partly due to inhibition of insulin secretion. glucagon and, with some molecules, delay gastric emptying. These molecules induce weight loss, which is mainly attributed to the direct effect of GLP-1 on appetite- regulatory neurons in the hypothalamus; Brain effects as well as mediated effects may also contribute under certain circumstances.
While short-acting GLP-1 receptor agonists show significant inhibition of gastric emptying, this effect may be attenuated in long-acting molecules. Nausea and vomiting are the most common side effects and may limit the dose and therefore the therapeutic effect. Molecules with a lower peak-to-trough ratio appear to have fewer GI side effects, and these side effects can be further managed through slow, gradual dose escalation leading to higher doses and better efficacy. These insights have led to the development of currently generated GLP-1 receptor agonists in which molecules with optimized pharmacokinetic properties are delivered through optimized dosing regimens. achieved dramatic glucose reductions and weight loss in patients with T2DM. Weight loss caused by the molecules was even more pronounced in people without diabetes, leading to the development of some of these molecules as obesity therapy.
Long-term cardiovascular outcomes studies, a requirement for T2DM Development Programs, have revealed additional benefits of GLP-1 agonists. The majority of GLP-1 receptor agonists show a cardiovascular benefit, as evidenced by a reduction in myocardial infarction, stroke, and cardiovascular mortality. Additionally, decreased albuminuria and decreased glomerular filtration rate were observed in some studies, raising the possibility of renal benefit (The underlying mechanisms of these benefits are unclear. -1 consistently showed increased heart rate and decreased blood pressure, as well as small improvements in serum lipid profile, Most notably a reduction in postprandial triglyceride involvement and small improvements in lipoproteins Low-density lipoprotein (LDL)- and high-density lipoprotein (HDL)-cholesterol, as well as anti-inflammatory effects, may contribute to the observed cardiovascular and renal profiles (Nauck et al., 2017). Initial concerns about potential safety issues with GLP-1 receptor analogues, including medullary carcinoma and pancreatitis, did not manifest as significant issues in the clinic (Nauck et al., 2017). Indeed, GLP-1 receptor agonists are now firmly established in the T2DM treatment paradigm and as the preferred treatment for patients with atherosclerotic disease, which patients will enjoy benefit from weight loss, and the individual needs to achieve excellent glucose control without the risk of hypoglycemia (American Diabetes Association, 2019). The next wave of innovation, currently underway, aims to develop bioavailable versions of GLP-1 receptor agonists to extend the benefits of GLP-1-based therapy to the next stage. front of the diabetes treatment model.
Các nghiên cứu đã chỉ ra rằng thụ thể GLP-1 có khả năng làm giảm nguy cơ mắc bệnh tim mạch như đột quỵ, nhồi máu cơ tim
GIP receptor agonists and GIP/GLP-1 . duality
Unlike GLP-1, very little research has been conducted to develop GIP as a standalone therapy. In people with T2DM, GIP has little effect on insulin secretion, leading to the concept of diabetes as a GIP-resistant state. While the molecular basis for GIP resistance is not well understood, it has been found to be strongly associated with hyperglycemia and the ability of GIP to induce insulin secretion may at least be partially restored by treatment. normalize glucose levels with insulin or sulfonylureas. Therefore, the combination of GIP with other glucose-lowering agents may help to unleash its therapeutic benefits. Studies of GIP transmission have informed our understanding of the physiology and pharmacology of GIP. Most prominently, GIP is known to increase circulating glucagon levels under hypoglycemic conditions, likely reflecting the direct effect of GIP on pancreatic alpha cells. GIP activates adipose tissue blood flow and increases glucose and fatty acid uptake into human adipose tissue with high levels of GIP receptor expression in adipose tissue. Unlike GLP-1, GIP has no effect on gastric emptying and no inhibition of food intake has been reported with GIP molecules administered peripherally or preclinically or in man.The ability of GIP to stimulate fatty acid uptake into adipose tissue, together with the observed weight loss in GIP or GIP receptor knockout mice, has led to the notion that GIP may be an obesity-inducing molecule , promotes the development of GIP receptor antagonists as a treatment for obesity. Indeed, antibodies to GIP or GIP receptors potently and selectively reduce body weight in mice and nonhuman primates. However, long-acting GIP agonist peptides did not induce weight gain in preclinical models, and weight loss was quite robust induced by GLP-1, challenging the assumption that GIP is a predisposing factor for obesity.
Observing the weight loss synergistic effect of GIP in combination with GLP-1 treatment in preclinical models has promoted the development of dual GIP and GLP-1 receptor agonists (also referred to as ' Twincretins Phone) for the treatment of T2DM and obesity. First molecule tested in humans showed significant glucose reduction and weight loss compared with placebo but did not differentiate itself significantly from the single GLP-1 receptor liraglutide agonist in early clinical studies. . Recently, a novel GIP and GLP-1 receptor agonist, (now known as tirzepatide), significantly improved glucose control and body weight in a phase 2b study when compared with dulaglutide, a GLP-1 receptor agonist. Tirzepatide lost up to > 15% weight in about one-quarter of patients, and normalized blood glucose (HbA1c less than 5.7%) in about one-third of patients, defining a new level of efficacy for T2DM therapy. Whether. The underlying mechanisms of tirzepatide's effectiveness are not fully understood. Compared with previous dual GIP/GLP-1 receptor agonists, tirzepatide has a greater potency for the GIP receptor than the GLP-1 receptor, providing a possible explanation for its profound clinical benefit. its color. Preclinical data demonstrate that both GIP and GLP-1 receptors contribute to the hypoglycemic effect of tirzepatide. Clinical research is ongoing to examine the mechanisms of tirzepatide's weight loss- and hypoglycemic effects in more detail. It will be interesting to see whether GIP-specific effects on adipose tissue blood flow and nutrient absorption contribute to the clinical efficacy of tirzepatide and how the recent role of Whether GIP in circuit appetite regulation in rats can modulate body weight in the context of GLP-1 action. Overall, tirzepatide demonstrates the potential of dual incretin therapy and is certain to spark additional studies on the effects of GIP. Tirzepatide Recently entered a phase 3 clinical trial for T2DM with additional plans to test its effects against obesity and non-alcoholic steatohepatitis (NASH).
OXM - Oxyntomodulin

Glucagon có thể làm tăng nồng độ glucose máu trong điều kiện hạ đường huyết
Interestingly, co-administration of glucagon and GLP-1 in preclinical studies resulted in weight loss beyond what could be achieved with GLP-1 alone while maintaining the glucose-lowering effects of GLP- first. In humans, similar effects can be achieved by administering OXM at doses expected to activate both glucagon and the GLP-1 receptor. In both preclinical and human studies, glucagon increased energy expenditure, albeit modestly, even in the presence of GLP-1. Furthermore, in preclinical models, lipid metabolism was profoundly improved compared with the use of a selective GLP-1 receptor agonist, suggesting a potential therapeutic benefit of NASH. These findings have sparked considerable interest in discovering a parent compound of glucagon and GLP-1, some of which are moving towards clinical trials. The most active molecules were SAR425899 and MEDI0382 (now known as cotadutide), which showed impressive weight loss in a short-term study in people with obesity or T2DM as well as reduced liver fat. The hypoglycaemic effect is similar to what would be observed with GLP-1 receptor agonists, suggesting that these molecules may balance GLP-1 and glucagon activity. It is important to note that to date there has been no direct comparison of glucagon/GLP-1 receptor dual agonist/Glucagon with a single human GLP-1 receptor agonist. While MEDI0382 was included in phase 2b studies for T2DM, obesity, and NASH, SAR425899, also another glucagon/GLP-1 receptor dual agonist, MRK-8521, has been discontinued, potentially. reflects the challenges in developing multifunctional glucagon/GLP-1 receptor agonists.
Concurrent with the development of similar dual agonists, efforts are underway to combine the pharmacological properties of GLP-1, GIP and glucagon for even greater weight loss. In preclinical models, these combinations can normalize the body weight of very obese mice, and similar effects can be achieved with the dual functional molecule. Triangles of GLP-1, GIP, and glucagon. Several triads of nonmolecular molecules are currently in development, with at least three in early clinical trials (PYY) One gut-derived peptide that could serve as a complement to GLP-1 is PYY .
PYY
PYY is a 36-amino acid peptide of the pancreatic polypeptide (PP) family, which includes PP secreted by the pancreas and neuropeptide Y (NPY) produced by neurons in the CNS. While including hairpins that are similar in modular structure and share the same G-protein-coupled NPY. The receptors (Y1, Y2, Y4 and Y5), PYY are mainly secreted from intestinal L cells and are functionally distinct from PP and NPY.PYY exists in two endogenous forms, PYY1-36 and PYY3-36, differences in abundance in fasting versus postprandial states and in selectivity for NPY receptors. Similar to GLP-1, PYY concentrations increased postprandial and correlated with sustained weight loss obtained after 8 weeks of a low-calorie diet that maintained weight loss for 1 year. PYY3-36 is produced from PYY1-36 through dipeptidyl peptidase IV (DPP4) activity and is the major postprandial circulating form of PYY. Unlike GLP-1, enzymatic cleavage of PYY1-36 by DPP4 does not reduce the affinity of PYY to a single receptor; instead, it improves selectivity. Thus, while PYY1-36 binds all NPY receptors, PYY3-36 acts as a selective Y2 agonist, thereby exhibiting its unique action.
Discovered in the early 1980s, first reports suggested a limited role for PYY in the gastrointestinal tract, including promoting gastric and pancreatic secretion and controlling intestinal motility. However, it has been found that PYY3-36 plays a role in energy homeostasis through a central effect as demonstrated by experiments where administration of exogenous PYY3-36 reduced feed intake in both rodents and humans and body weight reduction achieved in rodents, via the Y2 receptor as a potential target in the treatment of metabolic disease. Furthermore, there is evidence that PYY may play a role in insulin sensitivity and glucose uptake, likely through the Y2 receptor. While these mechanisms are still under intense investigation, the benefits of insulin-sensitizing effects for the treatment of metabolic diseases should not be overstated.
PYY and GLP-1 have been shown to co-exist in L cells secreting from the same secretory vesicle and appear to respond to similar stimuli, suggesting that PYY and GLP-1 play a complementary role. together. In addition, co-infusion of PYY3-36 and GLP-1 produces a synergistic effect of reduced feed intake in humans, and acute and chronic toxicity studies in rodents demonstrate additive and/or synergistic effects. consensus of PYY3-36 and GLP-1 on improvements in energy homeostasis. While gastrointestinal side effects may limit the use of Y2 receptor agonist PYY3-36 analogs as a therapeutic target, especially when combining a GLP-1 analog with a The long-acting PYY itself reduces food intake in non-human primates with minimal simulation, even when combined with liraglutide further reduces food intake. Several long-acting PYY3-36 analogs and Y2 receptor agonists are in early clinical trials and it is anticipated that these molecules will eventually be tested in combination with GLP analogs. -first. In addition, co-molecular approaches to PYY/GLP1 are under preclinical investigation.
Please follow the series on “Effects of the intestine in the treatment of metabolic diseases. Drugs and Future Prospects” by Dr. Nguyen Khanh Hoa:
Part 1: Effect of the gut in the treatment of metabolic diseases. Drugs and Future Prospects Part 2: Intestinal Peptide-Based Approaches Part 3: Other Enteric Peptides-Based Approaches Part 4: Is the gut really the primary source of enteric Peptides? Part 5: Activation of Nutritional Receptors in the Intestine as a Therapeutic Strategy Part 6: Brake Inhibition: Can Somatostatin Antagonists Be Useful for the Treatment of Diabetes?