This is an automatically translated article.
Article by Dr. Nguyen Khanh Hoa - Vinmec Institute of Stem Cell and Gene TechnologyThis article aims to discuss the variety of methods currently being developed that seek to take advantage of the gut as a new therapeutic approach with the hope that we can achieve the effectiveness of these therapies. surgical intervention with less invasive and more scalable solutions in the treatment of metabolic diseases.
1. Summary
Twenty-five years ago, the goals of obesity and diabetes treatment primarily focused on target organs known to be involved in the body's energy balance and glucose regulation, including the brain, muscles, tissues, and organs. fat and pancreas. Today, the most effective therapies are focused around the gut. This includes surgical options, such as longitudinal gastrectomy and Roux-en-Y gastrectomy, which can provide sustainable weight loss and diabetes relief while extending to pharmaceutical treatments simulate or amplify various signals originating from the gut.This article aims to discuss the variety of methods currently being developed that seek to take advantage of the gut as a new therapeutic approach with the hope that we can achieve the effect of surgical interventions with less invasive and more scalable solutions in the treatment of metabolic diseases. Local hormones of the intestine as well as hormones of the pancreas are focused on analyzing their potential as well as their value in clinical applications, some new active substances act on intestinal receptors, of a special group of cells. The intestine is responsible for the production of hormones for digestion and blood sugar balance. Based on those analyses, the author predicts a future revolution in the treatment of metabolic diseases based on the development of future enteral approaches.
Type 2 diabetes mellitus (T2DM) is a prominent and growing problem affecting nearly 10% of the US population (National Diabetes Statistical Report, 2017). Combined with the rapidly increasing prevalence of obesity, it is now estimated that approximately 40% of the U.S. adult population has a future likelihood of developing T2DM (National Health and Nutrition Investigations Commission. Survey, 2015 2016). These statistics are more alarming considering that T2DM and obesity are associated with a wide range of metabolic dysfunctions, including high blood pressure, dyslipidemia, heart failure, and non-alcoholic fatty liver cirrhosis. alcohol . While there is an increasing number of pharmacological treatments for T2DM and obesity, only about less than 50% of people with T2DM achieve their treatment goals and none of the currently available treatments prevent them. progression of treatment-directed diabetes leading to the need for insulin. Furthermore, the increased rate of T2DM will lead to an escalation in treatment costs and manpower for treatment.

Các nghiên cứu đã chỉ ra rằng tiểu đường tuýp 2 và béo phì có liên quan đến một loạt các loai rối loạn chức năng chuyển hoa
Currently, the most effective strategy to treat T2DM and obesity involves the gastrointestinal (GI) tract, or intestines, an outcome that would be nearly impossible to apply based on the research literature available from 25 years ago. The purpose of this article is to outline current treatment options based on enteric effects and to discuss for the next generation, based on enteral effects that have been or are being studied. We will also explore potential strategies to identify novel therapies based on gut effects or on gut-derived signaling systems.
2. Surgical intervention
Of the available treatments for obesity and T2DM, surgical interventions are clearly the most effective. The two most common types are Roux-en-Y gastro-intestinal bypass (RYGB) and longitudinal gastrectomy (VSG) (Figure 1). The clinical effects of both methods are impressive. In RYGB gastro-intestinal anastomosis, on average patients lose about 70% of their excess body weight in the first 12 months after surgery, with a similar outcome for longitudinal gastrectomy. However, what is actually achieved by surgical intervention that differs from other weight loss programs is endurance. In the case of RYGB, the Swedish Outcomes Study found that patients still lost 28% of their body weight after 14 years. This is in contrast to lifestyle interventions that had little overall benefit on weight after 5 years of the intervention.Both RYGB and VSG show profound improvements in glycemic control. In a randomized trial, RYGB or VSG surgery for T2DM patients was associated with a significantly greater reduction in mean HbA1C from baseline than in patients treated with antidiabetic therapy alone. 5 years (-2.1% vs - 0.3%, p = 0.003). This is a single study, but numerous trials and initial observational data on surgical intervention have shown that high rates of remission for type 2 diabetes can be achieved with medical therapy. These clinical effects have led to the mention of these methods as “metabolic surgery” when used primarily for the treatment of T2DM. This is highlighted in a recent statement helping to recognize metabolic surgery as the treatment of choice for type 2 diabetes. This leads to an important question: Is the effect strong Is T2DM a cumulative result primarily from weight loss that occurs as a result of surgery? This is a complex and controversial topic that is beyond the scope of this article. However, dramatic improvements in glycemic regulation were observed in the first few days after surgical intervention and before weight loss peaked almost 1 year after surgery. Furthermore, improved glucose regulation is not the only benefit, but a host of other obesity-related complications are reduced after surgical intervention, including cardiovascular risk factors, sleep apnea, and even obesity-related cancers. Overall, gastrointestinal remodeling with RYGB or VSG surgery has profound physiological effects leading to success in weight loss and improved glucose regulation.
Mechanical restriction and caloric malabsorption are often used to explain the powerful effects of metabolic surgery. Therefore, VSG is called a limited operation because it reduces the volume of the stomach by 80%, and RYGB is considered "restrictive", because the functional volume of the stomach is drastically reduced. However, RYGB is also ‘‘reduced’’ because the entire stomach area and the upper part of the small intestine are bypassed for reduced absorption. While a mechanistic explanation is likely to play some role, a full explanation of the postoperative improvements in energy and glucose homeostasis remains to be elucidated.
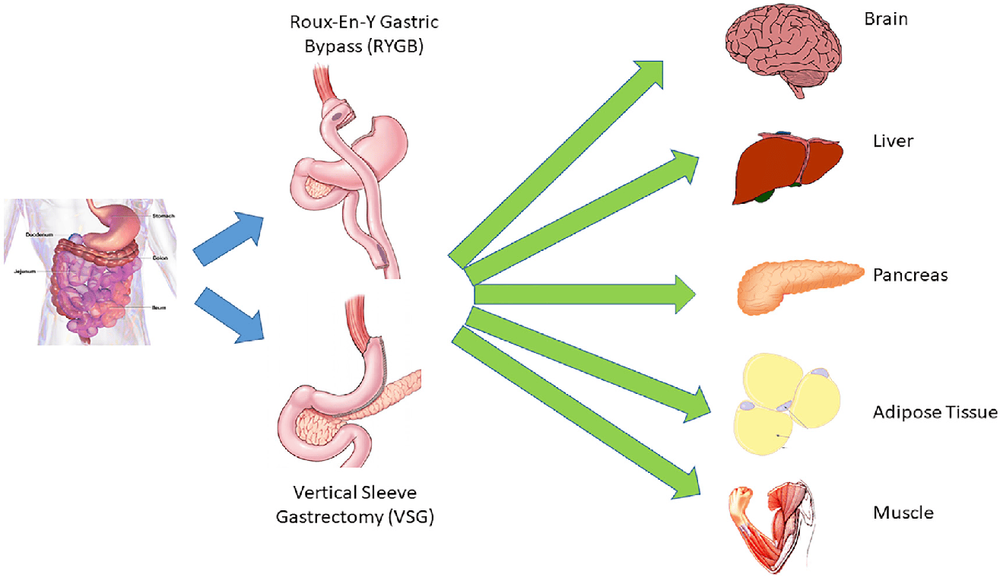
Tác dụng đa chiều của phẫu thuật giảm cân đối với nhiều hệ thống cơ quan
After surgery, the patients reported clearly less hunger despite the dramatic weight loss. If surgery does its job by preventing ingestion and/or absorption of calories, the patient will lose weight but will go hungry. So the notion that surgery works primarily by blocking calorie intake or absorption is simply inconsistent with the observed evidence.
Because mechanistic explanations are insufficient to explain the benefits of bariatric surgery, increased research has begun to focus on other mechanisms, notably neurons and hormone signaling from the gut. Although local distribution of the gut provides important signals back to the CNS that may alter both metabolism and appetite. There is no direct evidence that these signals are due to weight loss surgery.
Furthermore, while the vagus nerve may play a role in altering food choices after bariatric surgery, there is currently little evidence that such signals contribute to weight loss and improvement. blood glucose. While neural signaling from the gut appears to be less likely, it is clear that both VSG and RYGB alter the production of hormonal signals from gastrointestinal cells, including a number of strategic signaling pathways. validated therapy for T2DM and obesity, and has the potential to highlight future therapy. These include traditional intestinal peptides such as glucagon-like peptide 1 (GLP-1) and less traditionally represented hormones, bile acids.
While specific intestinal peptides will be discussed in the next section, Gastric bypass and esophagogastroduodenectomy also result in weight-independent increases in the levels and composition of bile acids. Bile acids can bind to and activate cell surface receptors called Takeda G protein-coupled receptor 5 (TGR5) and nuclear receptors called farnesoid X receptor (FXR). In turn, loss of FXR function significantly reduces the likelihood of gastric bypass surgery in terms of body weight or glucose tolerance, and several other studies link bile acid signaling with the benefit of surgery. weight loss. It is important to note that the source of these signals may not always be the host organism, as surgical procedures can greatly affect the composition of bacteria in the gut. These gut bacteria are also capable of producing a large number of nerve and hormonal signals that may also underlie the effects of surgery. In summary, it is unlikely that the sole factor or signal underlies the effectiveness of metabolic surgery. Rather, it is more likely a ‘‘polyphonic’ effect.
Những vi khuẩn đường ruột này cũng có khả năng tạo ra một số lượng lớn các tín hiệu thần kinh và nội tiết tố có thể cũng là nền tảng của tác dụng của phẫu thuật
Please follow the series on “Effects of the intestine in the treatment of metabolic diseases. Drugs and Future Prospects” by Dr. Nguyen Khanh Hoa:
Part 1: Effect of the gut in the treatment of metabolic diseases. Drugs and Future Prospects Part 2: Intestinal Peptide-Based Approaches Part 3: Other Enteric Peptides-Based Approaches Part 4: Is the gut really the primary source of enteric Peptides? Part 5: Activation of Nutritional Receptors in the Intestine as a Therapeutic Strategy Part 6: Brake Inhibition: Can Somatostatin Antagonists Be Useful for the Treatment of Diabetes?