This is an automatically translated article.
The article was written by Doctor, Dr. Le Xuan Luat - Infectious, hepatobiliary doctor - Department of General Internal Medicine, Vinmec Times City International General Hospital.Nevirapine was approved for use in 1996 in the US. Nevirapine belongs to the class of non-nucleotide reverse transcriptase inhibitors (NNRTIs). After the HIV virus enters the host cell, the HIV RNA strand is released, and the single strand RNA is reverse transcribed to form DNA by reverse transcriptase. The drug inhibits the formation of DNA, reduces the amount of HIV virus.
1. First-line ARV regimens
Table 1: First-line ARV regimens| Đối tượng | Phác đồ ưu tiên | Phác đồ thay thế | Phác đồ đặc biệt (khi không dùng được hoặc không có phác đồ ưu tiên hay thay thế) |
| Người lớn bao gồm cả phụ nữ mang thai (*) và trẻ từ 10 tuổi trở lên | TDF + 3TC (hoặc FTC) + DTG1 | TDF + 3TC + EFV 400 mg |
TDF + 3TC (hoặc FTC) + EFV 600mg AZT + 3TC + EFV 600 mg TDF + 3TC (hoặc FTC) +PI/r ABC + 3TC + DTG1 |
| Trẻ dưới 10 tuổi | ABC+ 3TC + DTG | ABC+3TC+ LPV/r |
ABC + 3TC + EFV3 (hoặc NVP) AZT + 3TC + EFV3 (hoặc NVP) AZT + 3TC + LPV/r (hoặc RAL) |
| Trẻ sơ sinh (trẻ dưới 4 tuần tuổi) | AZT+3TC+RAL | AZT+3TC+NVP | AZT+3TC+LPV/r |
| Tên thuốc | Viết tắt |
| Tenofovir | TDF |
| Efavirenz | EFV |
| Lamivudine | 3TC |
| Abacvir | ABC |
| Dolutegravir | DTG |
| Nevirapine | NVP |
| Lopinavir/ ritonavir | LPV/r |
| Raltegravir | RAL |
2. Nevirapine (NVP) 1st line ARV side effects
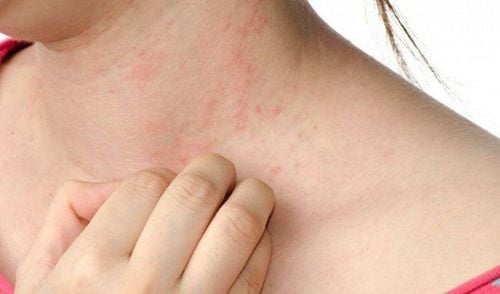
Rash The most common side effect of Nevirapine is a rash. The incidence of mild rash in nevirapine users can be as high as 50%. Nevirapine users may develop a rash at any time during the first 4-6 weeks of taking the drug. The first 10 days of taking the drug is the time when the rash is most common. The rash may appear slowly and spread to the whole body. The rash can be mild to severe, ranging from erythema, papule, vesicular vesicles, mucosal lesions of natural cavities.Liver damage NVP causes liver damage to varying degrees. NVP users need periodic liver enzyme monitoring. If liver enzymes are elevated above 5 times normal, consideration should be given to discontinuing NVP.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.