This is an automatically translated article.
The article is written by Master, Doctor Mai Vien Phuong - Gastroenterologist - Department of Medical Examination & Internal Medicine - Vinmec Central Park International General Hospital.
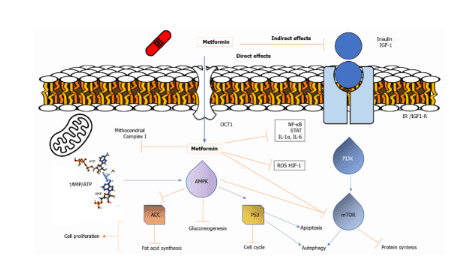
Metformin can directly activate AMPK, leading to inhibition of downstream Akt/mTOR signaling and inhibition of cell proliferation. Among pancreatic cancer patients, being treated with metformin seems to be able to effectively improve symptoms.
1. Metformin acts as an anti-cancer agent
Two potential mechanisms for the anticancer activity of metformin have been proposed. Specifically:
Metformin can directly activate AMPK, leading to inhibition of downstream Akt/mTOR signaling and inhibition of cell proliferation. The decrease in circulating insulin and IGF levels caused by metformin may reduce the activation of the IGF receptor signaling axis, resulting in decreased growth promotion and mitotic formation. Thus, the antitumor effect of metformin is shown to be mediated by an improved metabolic system or directly on tumor cells.
2. Metformin and pancreatic cancer
Pancreatic cancer is the fourth leading cause of cancer death in the United States and its prognosis is still very low, encouraging research to find positive agents of improvement in treatment is an urgent need that has not been met. Pancreatic carcinoma (PDAC) is the most common histological type. The association between metformin use and reduced incidence of pancreatic cancer in patients with type 2 diabetes was first recognized by two large clinical studies.
In an extensive practice retrospective cohort, Currie et al. reported a reduced risk in metformin users in relation to sulfonylurea (HR: 0.20; 95% CI: 0.11-0.36). and in humans on insulin-based therapy (HR: 0.22; 95% CI: 0.12-0.38). Similarly, in a hospital-based case-control study, Li et al reduced risk in metformin users compared with nonusers (OR: 0.38; 95% CI: 0.21- 0.67). Several meta-analyses have strongly supported a reduction in pancreatic cancer risk with metformin use in patients with type 2 diabetes. However, this effect needs to be confirmed in prospective clinical trials. great.
Trắc nghiệm: Bạn biết gì về các yếu tố nguy cơ, chẩn đoán và điều trị ung thư tuyến tụy?
Ung thư tuyến tụy phổ biến thứ 10 trong những bệnh ung thư mới và là nguyên nhân thứ 4 gây tử vong do ung thư ở nam, nữ. Bài trắc nghiệm này sẽ kiểm tra kiến thức của bạn về các yếu tố nguy cơ, chẩn đoán và cách điều trị ung thư tuyến tụy.
Bài viết tham khảo nguồn: medicalnewstoday 2019
3. Survival prognosis of pancreatic cancer patients using metformin
In a retrospective study, Sadeghi et al reported a 36% lower risk of death (HR: 0.64; 95% CI: 0.48–0.86), a survival benefit of 4 months (15.2 months vs 11.1 months) and an approximately 2-fold increase in 2-year survival (30.1% vs 15.4%) in patients receiving metformin compared with patients receiving internal stay unused. Interestingly, longer survival was observed only in non-metastatic disease, when stratified by disease stage. Other evidence of improved survival was mentioned in the resection subgroup or local progression but no metastasis. Specifically, among PDAC-treated patients, metformin use appeared to improve OS after 18 months. With regard to locally advanced or metastatic disease, other conflicting evidence on survival in PDAC patients with metformin exposure was reported in only one Asian cohort.
4. What do the studies say?
A large meta-analysis analyzed data from 12 retrospective cohorts demonstrating OS improvement in metformin users over different periods (HR: 0.77; 95% CI: 0, 0. 68-0.87). Recently, a meta-analysis, including two RCTs, reanalyzed improvement in OS and confirmed benefit across the entire population of diabetic patients with PDAC (HR: 0.86; CI 95 %: 0.76-0.97). Subgroup analysis in this study showed improved survival in patients with resected or locally advanced tumors, but not in the metastatic group. Similar results were observed in another group with an overall survival benefit at different stages, which was more pronounced in the less advanced stage and disease subgroups. Asian people.
5. Outcomes in second-line pancreatic cancer patients
Considering second-line therapy, a prospective, single-arm study failed to achieve metformin survival relative to paclitaxel. The results of recent clinical trials are expected with considerable interest. NCT01666730 explores the FOLFOX6-related improvement in overall survival of metformin in patients with metastatic disease. NCT02005419 evaluated 1-year DFS with the combination of metformin, gemcitabine in resected subjects, and NCT02048384 analyzed the safety of metformin with or without rapamycin after disease stabilization on first-line chemotherapy for metastases.
This clinical evidence relates to preclinical data, that pancreatic cancer cells are sensitive to inhibition of oxidative phosphorylation, decreased insulin-IGF signaling, and inhibition of the mTOR pathway through activation AMPK, these are some of the main anticancer effects of metformin. Identification of predictors or prognostic factors of response to metformin should be appropriate to select patients most likely to benefit from metformin. Recent advances in molecular characterization that can differentiate differential biology and response to therapy in patients with morphologically similar PDACs can be included in clinical trials.
Furthermore, the recent empiric challenge of the standard of care in advanced pancreatic cancer treatment with multi-chemotherapy also offers new perspectives, as patients experience longer survival times with higher requires a combination of other active ingredients. Future trials will include disease staging, biomarker identification, and metformin concentrations in cancer tissue to robustly assess the benefit of metformin in the treatment of PDAC.
6. Pancreatic neuroendocrine tumor
Another type of pancreatic cancer with an increased incidence are pancreatic neuroendocrine tumors (panNETs). Few studies have evaluated the clinical benefit of metformin in the treatment of panNET. According to Pusceddu et al., in a multicenter retrospective cohort of patients receiving everolimus with or without somatostatin analogues, an increased PFS was reported in diabetics exposed to metformin compared with diabetic patients without exposed or non-diabetic patients [44.2 vs 20.8 months (heart rate: 0.49; 95% CI: 0.34-0.69) or 15.1 months (HR: 0.45) ; 95% CI: 0.32-0.62), respectively]. This result correlates with in vitro evidence that metformin reduces proliferation in human panNET cell lines.
A recent study demonstrated that the combination of metformin and everolimus strongly inhibited human panNET cell proliferation through mTOR inhibition, compared with either agent used alone. The results of the ongoing prospective trial NCT02294006 are expected to better assess the impact of this experimental treatment on PFS at 12 months.
7. Conclusion
Remarkable intracellular pathway alterations induced by carcinogenesis, potential mechanisms of metformin antitumour activity have been supported. They have revealed newly discovered target molecules and therapeutic possibilities. With regard to epidemiological, preclinical, and clinical studies, data support the view that metformin is beneficial for some patients with gastrointestinal tumours, requiring rigorous clinical trials to determine the benefits of metformin. who may benefit from the combination of metformin.
However, because survival outcomes are influenced by a multitude of factors, such as cancer type, differentiation, stage and treatment, to reposition the use of metformin in gastrointestinal cancer, It is essential to consider the characteristics of the patient. This could serve as a biomarker that predicts the anti-tumor effects of metformin, such as insulin resistance, diabetes, body composition, and chronic diseases related to inflammation as well as carcinogenic pathways. specific tumor-driven cancers, which may interfere with the direct and indirect antitumor effects of metformin. Currently, early cancer screening is considered the perfect measure in timely detection and treatment of cancers. Reduce the cost of treatment and especially reduce the mortality rate in patients. Vinmec International General Hospital always deploys and introduces to customers the Early Cancer Screening Package at Vinmec - Peace of mind to live well to help with gene testing, imaging, testing of biomarkers to detect tumors you early.
Choosing the Early Cancer Screening Package at Vinmec - Peace of mind at Vinmec, customers will get:
Only one gene test can assess the risk of 16 common cancers in both men and women ( Lung cancer, colorectal cancer, breast cancer, pancreatic cancer, cervical cancer, stomach cancer, prostate cancer, ....) Early detection of early signs of cancer cancer through imaging, endoscopy, and ultrasound. The operation is simple, careful and accurate. A team of well-trained specialists, especially in oncology, are capable of handling cancer cases. With a system of facilities, advanced and modern medical equipment and a team of doctors with deep expertise and experience, it will help the examination and treatment process of patients at Vinmec become faster with High efficiency, save cost and time.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References: Cunha Júnior AD, Bragagnoli AC, Costa FO, Carvalheira JBC. Repurposing metformin for the treatment of gastrointestinal cancer. World J Gastroenterol 2021; 27(17): 1883-1904 [DOI: 10.3748/wjg.v27.i17.1883]