This is an automatically translated article.
The article was written by Dr. Hoang Minh Duc - Team Leader of Experimental Production Project, Research Institute of Stem Cells and Gene Technology VinmecThe storage of BM-MSC, AD-MSC, and UC-MSCs stem cell lines as a source of cells for future use as needed is an appropriate and viable customer need.
3. Overview of umbilical cord mesenchymal stem cells
3.1 Structure and morphology of the umbilical cord
During human embryonic development, the umbilical cord is formed from the embryonic membrane after fertilization and the ureteral membrane becomes a special connection between the fetus, the placenta and the mother. The umbilical cord contains a viscous mucus that was discovered by Thomas Wharton and is commonly known as Wharton's jelly (WJ) [6].
The main function of this mucous membrane is to protect the blood vessels inside the umbilical cord (including 2 veins and 1 artery) as well as prevent obstruction and twisting of the umbilical cord. The mucous membrane is composed mostly of glycosaminoglycans, especially hyaluronic acid and chondroitin sulfate compounds [7].
In addition to the above compounds, the umbilical cord is composed mostly of collagen fibers that form a network of fibers that run the length of the umbilical cord, increasing the flexibility, strength and integrity of the structure. The cellular components that make up the umbilical cord are mostly mesenchymal cells such as fibroblasts, muscle cells, smooth muscle cells, and mesenchymal stem cells. Unlike other tissues in the human body, the WJ layer does not contain a network of capillaries or small blood vessels [8].
According to studies on embryonic development, the process of developing the vascular system and regenerating blood inside the umbilical cord starts from the 6th week of embryogenesis, but from the 7th to the 9th week During pregnancy, this process stops and capillaries begin to gradually disappear throughout the umbilical cord structure.
3.2 Umbilical cord mesenchymal stem cells (TBGTMDR)
Research on the umbilical cord began with the discovery of hematopoietic stem cells from cord blood itself in 1974, but at that time the cord was considered a medical waste and had no value. scientific treatment [9].
However, this view was completely abandoned in 1991 when McElreavey et al. successfully isolated fibroblast-like cells from the Wharton's Jelly mucosa and performed biological characterization analysis. of these cell lines [10].
In 2004, the biological properties of filamentous umbilical cord cell lines were demonstrated to be mesenchymal stem cells through their proliferation, differentiation, and surface markers such as CD29, CD44, and CD51. , CD73, CD90 and CD105.
Most importantly, these cells do not have hematopoietic stem cell surface markers such as CD34 and CD45 [11].
The above properties are completely similar to the concept of mesenchymal stem cells prescribed by the International Society for Cell Therapy (ISCT) [12].
Currently, TBGTMDR can be isolated from whole sections of the umbilical cord or from individual components of the umbilical cord such as stem cells obtained from arteries and veins, umbilical cord membranes and from masses WJ slime.
![Hình 1: Các tế bào gốc trung mô được tìm thấy tại các cấu phần khác nhau của dây rốn [13].](/static/uploads/20200915_130137_157677_screenshot_16001748_max_1800x1800_png_fe1450d86c.png)
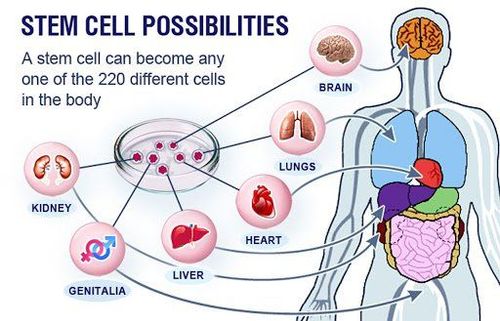
4. Applications of mesenchymal stem cells
Worldwide, FDA has allowed clinical trials (phases 1-3) to evaluate the safety and effectiveness of using mesenchymal stem cells in the treatment of clinical diseases.
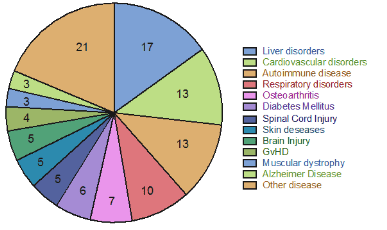
According to clinicaltrial.gov website, mesenchymal stem cells have been used to treat over 374 diseases ranging from metabolic disorders, immune system disorders to diseases such as Alzheimer's, neurological injuries , cardiovascular diseases (according to Clinicaltrial.gov statistics, https://clinicaltrials.gov/) with more than 115 independent studies worldwide (Figure 2).
In the above clinical studies, the infusion of mesenchymal stem cells into the patient's body did not show any adverse effects on the patient's health, except for some cases of patients with mild fever, or vomit.
Studies on stem cells usually focus on allogeneic Stem Cell Transplantation using autologous mesenchymal stem cells from bone marrow and adipose tissue because of the storage of umbilical cord mesenchymal stem cells. has just begun in recent years as cell culture and isolation techniques are being developed.
Therefore, very few patients are able to store their umbilical cord for use for autologous transplantation. However, there are still studies comparing the efficacy between autologous stem cell therapy and allograft.
Currently, at the Research Institute, mesenchymal stem cells from bone marrow and adipose tissue have been used to evaluate the safety and effectiveness in the treatment of diabetes and hormonal disorders in middle age. . Preliminary results showed that the cultured stem cell mass did not cause any adverse reactions or events.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References:
Friedenstein, A.J., J.F. Gorskaja, and N.N. Kulagina, Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol, 1976. 4 (5): p. 267-74. Sadan, O., E. Melamed, and D. Offen, Bone-marrow-derived mesenchymal stem cell therapy for neurodegenerative diseases. Expert Opin Biol Ther, 2009. 9 (12): p. 1487-97. Morrison, S.J. and D.T. Scadden, The bone marrow niche for haematopoietic stem cells. Nature, 2014. 505 (7483): p. 327-34. Pittenger, M.F., et al., Multilineage potential of adult human mesenchymal stem cells. Science, 1999. 284 (5411): p. 143-7. Kappy, N.S., et al., Human Adipose-Derived Stem Cell (ASC) Treatment Modulates Cellular Protection in Both in Vitro and in Vivo Traumatic Brain Injury Models. J Trauma Acute Care Surg, 2017. SPEERT, H., Obstetric-gynecologic eponyms; Thomas Wharton and the jelly of the umbilical cord. Obstet Gynecol, 1956. 8 (3): p. 380-2. Majore, I., et al., Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev, 2011. 7 (1): p. 17-31. Donders, R., et al., Human Wharton's Jelly-Derived Stem Cells Display a Distinct Immunomodulatory and Proregenerative Transcriptional Signature Compared to Bone Marrow-Derived Stem Cells. Stem Cells Dev, 2018. 27 (2): p. 65-84. Knudtzon, S., In vitro growth of granulocytic colonies from circulating cells in human cord blood. Blood, 1974. 43 (3): p. 357-61. McElreavey, K.D., et al., Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton's jelly portion of human umbilical cord. Biochem Soc Trans, 1991. 19 (1): p. 29S. Can, A., F.T. Celikkan, and O. Cinar, Umbilical cord mesenchymal stromal cell transplantations: A systemic analysis of clinical trials. Cytotherapy, 2017. 19 (12): p. 1351-1382. Galipeau, J., et al., International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy, 2016. 18 (2): p. 151-9. Cell4life. The benefits of storing umbilical cord tissue stem cells . 2016 [cited 2018 10 March]; Available from: cells4life.com