This is an automatically translated article.
The article was written by Dr. Hoang Minh Duc - Team Leader of Experimental Production Project, Vinmec Research Institute of Stem Cells and Gene Technology
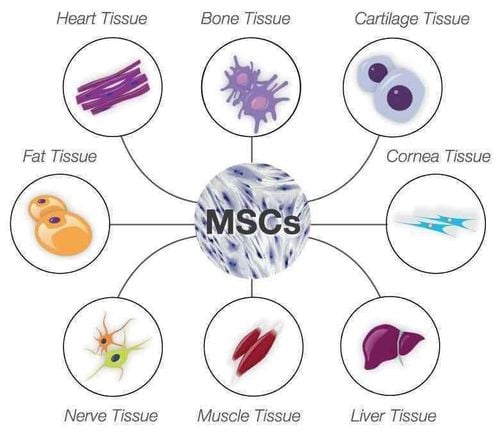
Mesenchymal stem cells are stem cells that possess special biological recognition properties such as the ability to proliferate when cultured outside the body (exogenous stem cells) and the ability to differentiate into a different types of cells such as bone, cartilage, fat, nerve, etc.
In the human body, mesenchymal stem cells can be found in many organs but are most abundant in bone marrow, adipose tissue, and umbilical cord. In terms of application, bone marrow derived mesenchymal stem cells (BM-MSC), adipose derived mesenchymal stem cells (AD-MSC), and Umbilical cord derived mesenchymal stem cells (UC-MSCs) are the three most widely used stem cell lines in areas such as knee treatment, cerebral palsy, bronchopulmonary dysplasia, and pulmonary embolism. chronic obstructive pulmonary disease (COPD), diabetes, facial rejuvenation, etc.
These cell lines show safety after stem cell transplantation and are effective in related diseases to the nervous system and lungs due to its immunomodulatory properties (reduces inflammation, reduces stimulation of the immune system in inflammatory areas, inhibits immune cells, etc.) and secretes Produce growth stimulants as well as extracellular microelements that support the regeneration of the nervous system, and stimulate the growth of endogenous stem cells.
Therefore, it is appropriate and feasible to store BM-MSC, AD-MSC, and UC-MSCs stem cell lines as a source of cells for future use as needed. customer.
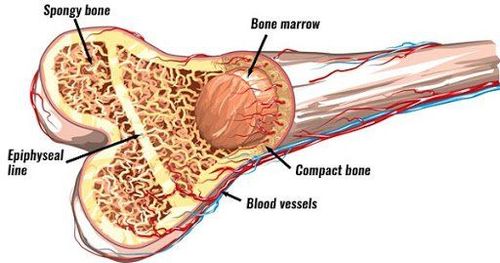
1. Mesenchymal stem cells from bone marrow
Since the 1950s, bone marrow has been described in the medical literature as a rich source of hematopoietic stem cells and a mixture of supporting cells for the production of hematopoietic components. In the 1960s, two famous scientists, Ernest A. McCulloch and James E. Till, carried out experiments on culturing bone marrow solutions with the results showing the ability of cells to form clusters in the bone marrow. first].
This experiment was the basis for a follow-up experiment performed by Friedenstein in the early 1970s that demonstrated the ability to proliferate from clusters after ex vivo culture of bone marrow cells. A series of experiments were performed and showed the proliferative ability of bone marrow cells and the ability to differentiate into a number of endemic cells such as bone, cartilage, and fat cells. Since then, the concept of mesenchymal stem cells was born with the scientific name of Mesenchymal stem cells (MSCs) [2].
In bone marrow, the population of mesenchymal stem cells is very small with the proportion accounting for 0.001 to 0.01% of the number of monocytes [3]. Although the number of mesenchymal stem cells is very small, under appropriate culture conditions, this cell line still has outstanding proliferative capacity and good differentiation ability.
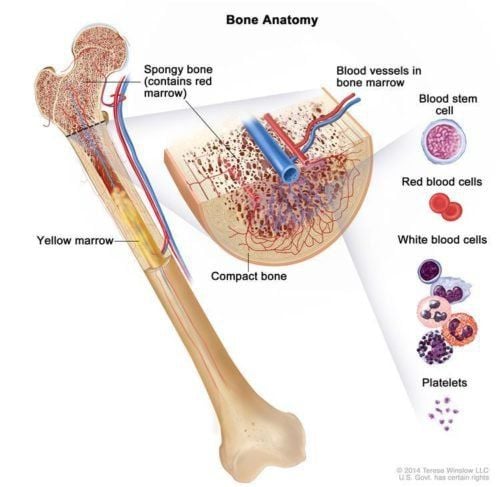
Bone marrow mesenchymal stem cells have so far been shown to be a pluripotent stem cell line, with limited differentiation potential in mesenchymal cell lines such as bone, cartilage, and adipocytes. . However, some other experiments also show that this stem cell line also has the ability to differentiate into liver cells, heart cells, nerve cells but the differentiation process has many stages and has a high success rate. low work.
To increase the possibility of isolating mesenchymal stem cells from bone marrow, the isolation of mononuclear cells present in bone marrow is one of the simple, inexpensive, and time-saving methods.
Among them, the method of collecting mononuclear cells by Ficoll density centrifugation technique is the most popular method. The basic principle of this technique lies in the process of separating different types of cells within the bone marrow based on their density when centrifuged with Ficoll [4].
After centrifugation, erythrocytes, neutrophils, and bone deposits will be in the bottom layer of the centrifuge tube, the monocyte layer will be in the middle layer between Ficoll and plasma, aka. is the Buffy coat.
At the start of culture (Passage 0 – P0), bone marrow mesenchymal stem cells have morphological characteristics similar to fibroblasts with small size, long polarized two ends , has the ability to multiply and duplicate at a slow rate.
The morphology of these cells usually becomes homogeneous and proliferates more rapidly through subcultures. Once the cell line is stable, mesenchymal stem cells from the bone marrow can be transplanted 10 to 20 times, depending on the nature of the cells, which are often directly related to the donor condition such as age, pathology, v...v...
2. Mesenchymal stem cells from adipose tissue
Knowledge about adipose tissue mesenchymal stem cells is mostly derived from basic biological studies of adipose tissue and their function inside the human body.
Adipose tissue is the type of tissue underlying the skin, or surrounding internal structures in the body, with the function of protecting, retaining heat, and storing energy for the body. There are two types of adipose tissue found including brown adipose tissue and white adipose tissue. Brown adipose tissue is adipose tissue with a specialized function and is found in abundance in infants to retain body heat.
Brown adipose tissue contains many mitochondria with great metabolic capacity and is rarely seen in adults except around the neck. In adults, the majority of white adipose tissue is located under the skin.
Adipose tissue is derived from the mesenchymal part of human development and is a rich source of mesenchymal stem cells. In adipose tissue, the process of fat formation (adiposegenesis) includes the process of maturation of fat cells, and the process of forming adipose tissue inside the body. To be able to complete this process, mesenchymal stem cell function plays a very important role because mesenchymal stem cells maintain their ability to differentiate into mature adipocytes.
Similar to bone marrow, the mesenchymal stem cell line is also found in adipose tissue with similar properties such as the ability to proliferate and differentiate (pluripotency). Adipose tissue that has been collected through surgical collection of adipose tissue or through liposuction is treated with collagenase enzymes to collect the Stromal-vascular cell fraction (SVF) [5]. .
This basal segment contains endothelial cells, fibroblasts, red blood cells, white blood cells, macrophages, pericytes, and mesenchymal stem cells.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References:
Friedenstein, A.J., J.F. Gorskaja, and N.N. Kulagina, Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol, 1976. 4 (5): p. 267-74. Sadan, O., E. Melamed, and D. Offen, Bone-marrow-derived mesenchymal stem cell therapy for neurodegenerative diseases. Expert Opin Biol Ther, 2009. 9 (12): p. 1487-97. Morrison, S.J. and D.T. Scadden, The bone marrow niche for haematopoietic stem cells. Nature, 2014. 505 (7483): p. 327-34. Pittenger, M.F., et al., Multilineage potential of adult human mesenchymal stem cells. Science, 1999. 284 (5411): p. 143-7. Kappy, N.S., et al., Human Adipose-Derived Stem Cell (ASC) Treatment Modulates Cellular Protection in Both in Vitro and in Vivo Traumatic Brain Injury Models. J Trauma Acute Care Surg, 2017. SPEERT, H., Obstetric-gynecologic eponyms; Thomas Wharton and the jelly of the umbilical cord. Obstet Gynecol, 1956. 8 (3): p. 380-2. Majore, I., et al., Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev, 2011. 7 (1): p. 17-31. Donders, R., et al., Human Wharton's Jelly-Derived Stem Cells Display a Distinct Immunomodulatory and Proregenerative Transcriptional Signature Compared to Bone Marrow-Derived Stem Cells. Stem Cells Dev, 2018. 27 (2): p. 65-84. Knudtzon, S., In vitro growth of granulocytic colonies from circulating cells in human cord blood. Blood, 1974. 43 (3): p. 357-61. McElreavey, K.D., et al., Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton's jelly portion of human umbilical cord. Biochem Soc Trans, 1991. 19 (1): p. 29S. Can, A., F.T. Celikkan, and O. Cinar, Umbilical cord mesenchymal stromal cell transplantations: A systemic analysis of clinical trials. Cytotherapy, 2017. 19 (12): p. 1351-1382. Galipeau, J., et al., International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy, 2016. 18 (2): p. 151-9. Cell4life. The benefits of storing umbilical cord tissue stem cells . 2016 [cited 2018 10 March]; Available from: cells4life.com