This is an automatically translated article.
Influenza is a contagious respiratory illness caused by influenza viruses that infect the nose, throat, and sometimes the lungs. It can cause mild to severe illness, and can lead to death. The best way to prevent the flu is to get a flu shot every year. The flu vaccine helps the body create an immune response against the flu virus. Viral antigens include toxins, chemicals, bacteria, viruses, or other substances that come from outside the body. Body tissues and cells, including cancer cells, also have antigens that trigger an immune response. These antigens can also be used as markers in laboratory tests to characterize those tissues or cells. In this article, we will provide useful information about the antigenic characteristics of influenza viruses.
1. Antigen of influenza virus
Antigen is a substance that is able to stimulate an immune response, especially activating lymphocytes, which are white blood cells that fight infections in the body. In general, two major divisions of antigens are recognized: external (or heterozygous) antigens and self-antigens. External antigens originate from outside the body. Examples include parts or substances produced by viruses or microorganisms (such as bacteria and protozoa), as well as substances in snake venoms, certain proteins in food and components of blood. bars and red blood cells from other individuals. Self-antigens, which originate from within the body. Normally, the body can make its own antigens, but in people with autoimmune disorders, the disorder triggers an immune response, leading to the production of autoantibodies. An antigen that induces an immune response, i.e., stimulates lymphocytes to produce antibodies or to attack the antigen directly is called an antibody.An antigen is a molecular structure on the surface of a virus that is received by the body's immune system and is capable of triggering an immune response (production of antibodies). On influenza viruses, major antigens are found on the surface proteins of the virus (see Figure 1).
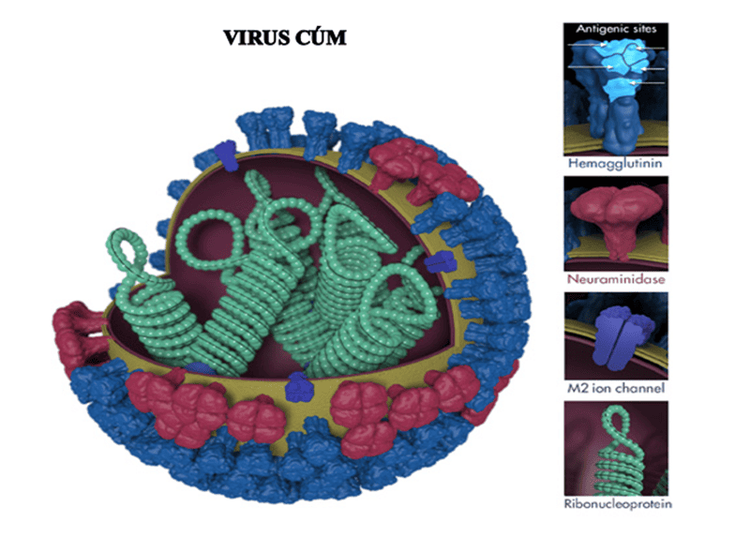
Hình 1. Các tính năng của virus cúm
When someone is exposed to the flu virus (through infection or vaccination), their immune system creates specific antibodies against the antigens (surface proteins) on that particular flu virus . The term "antigen property" is used to describe antibodies or immune responses triggered by antigens on a particular virus. “Antigen characterization” refers to the analysis of the antigenic properties of one virus to help assess its relevance to other viruses.
The image above shows various features of the influenza virus, including the surface proteins hemagglutinin (HA) and neuraminidase (NA). After being infected with the flu or receiving a flu vaccine, the body's immune system can develop antibodies that recognize and bind to antigen sites, which are regions found on the surface protein of the flu virus. . By binding to these antigenic sites, antibodies neutralize the flu virus, preventing the virus from growing in the body.
The important antigens of the influenza virus are located in the core and the outer shell.
The core of the influenza virus contains an RNA and protein molecule, corresponding to the S (Soluble) antigen. Although carrying the entire genetic code of the virus, this antigen has no meaning for the body's protective immune mechanism. The outer shell contains two important antigens, hemagglutinin (H) and neuraminidase (N). The H antigen is also known as red blood cell agglutination factor. This antigen helps the virus to attach to the cells of the respiratory mucosa, thereby entering the cells. It can adhere to the membranes of red blood cells in humans and some animals, causing these red blood cells to stick together to form a membrane at the bottom of the test tube - This is red blood cell agglutination. Antibodies corresponding to the H antigen, also known as erythrocyte agglutinin antibodies, have a protective effect. The N-antigen has enzymatic activity that thins the mucus in the respiratory tract, making it easier for the virus to contact the cells of the mucosa. In addition, it helps the virus to enter the cell easily, helps the assembly of virus components and exits the cell. Antibodies corresponding to the N antigen also have a protective effect on the body.
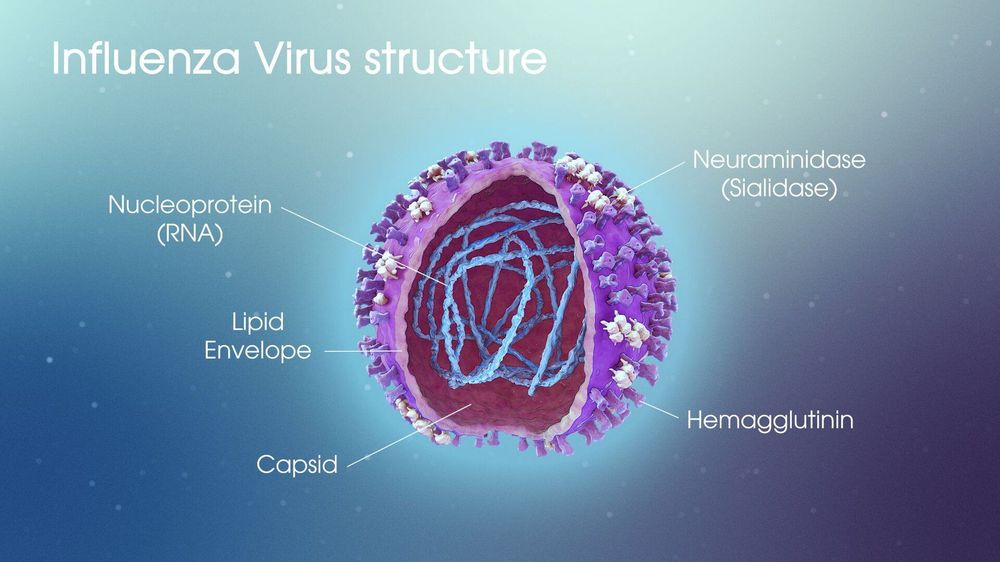
Phần vỏ ngoài virus cúm chứa hai kháng nguyên quan trọng là H và N
Two antigens H and N determine the pathogenicity of influenza viruses and are type-specific. However, the H and N structures can change into new H and N. So far, 13 H antigen structures have been detected, with symbols from H1 to H13 and 9 structures with N antigens with symbols from N1 to N9.
CDC has antigenically characterized approximately 2,000 influenza viruses each year to compare circulating influenza virus levels and monitor changes in circulating influenza viruses. Antigenic properties may indicate the ability of influenza vaccines to induce an immune response against circulating influenza viruses in humans. This information also helps experts decide which viruses should be included in the upcoming flu vaccine. Other information that determines the prevalence of influenza or other viruses is the result of serological tests and genetic sequencing.
2. Hemagglutinin Inhibition Test (HI Test)
Scientists use a test called the hemagglutinin inhibition (HI) test to characterize the influenza virus. The HI test works by measuring the level of antibodies that bind to the influenza virus.
Scientists use the HI test to assess antigenic similarity between influenza viruses. This test is especially useful to help select the viral vaccine to be used in the seasonal flu vaccine. HI test results can tell us whether antibodies developed against vaccination with one virus are antigenically similar enough to another circulating influenza virus to induce a response. immunity against the circulating virus. Scientists also use the HI test to compare antigenic changes in influenza viruses currently circulating with influenza viruses that have circulated in the past.
The HI test consists of three main components: antibodies, influenza virus and red blood cells mixed together of a microtiter plate. (See Figure 2.)
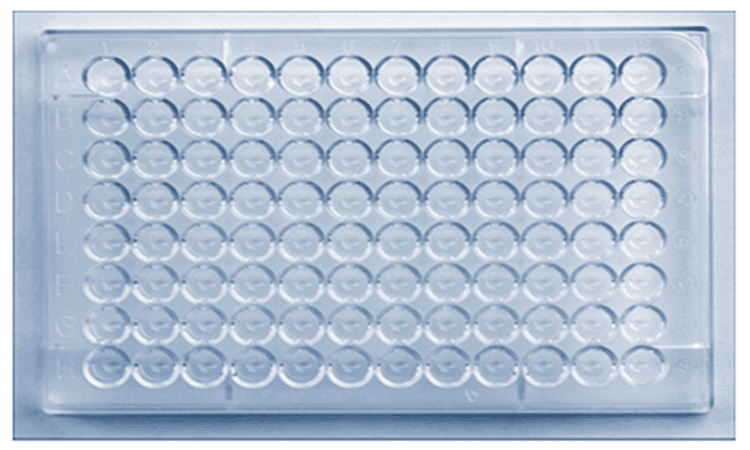
Hình 2. Một tấm Microtiter
A microtiter plate is used to perform the HI test. The dish contains small cavities that hold a small amount of liquid in which an antibody solution, influenza virus and red blood cells are inserted and allowed to interact. These recesses are arranged in rows and columns (identified on the microtiter plate by letters and numbers, respectively). The rows of plates can be used to test different influenza viruses against the same set of antibodies. Columns can be used to distinguish between larger dilutions, as a low-to-high scale goes from left to right (see for example Figure 3).
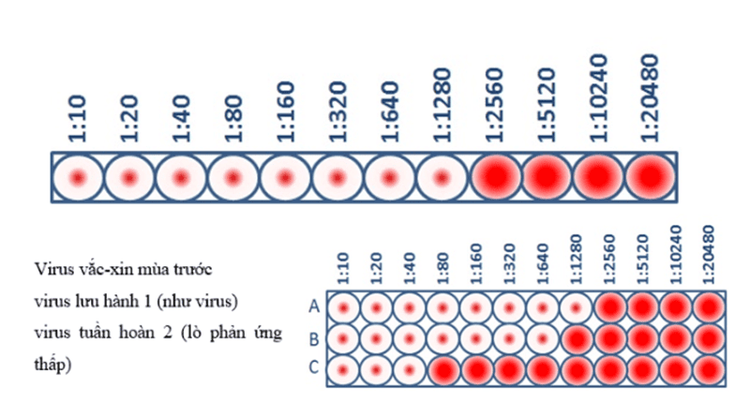
Hình 3. Xét nghiệm HI Titer và xét nghiệm đặc tính kháng nguyên
This virus sample has an HI of 1280, which means that the maximum dilution of the antibody that still blocks blood clotting is at a dilution of 1280. At this dilution, the antibodies are still capable of blocking blood clotting. ability to recognize and bind to viral antigens.
When CDC has antigenic specificity of influenza viruses to inform decisions about the formulation of a seasonal influenza vaccine, the HI test is used to compare circulating viruses (influenza type B & C viruses) currently with influenza type A virus vaccines. This allows scientists to quickly determine if a virus used in the seasonal flu vaccine is antigenically similar to circulating influenza viruses, and thus capable of generating an immune response against them.
The antibodies used in the HI test are obtained by injecting animals (usually ferrets) that have not been exposed to any influenza virus or vaccine earlier in life. The other animal's immune system produces antibodies in response to the antigens on the surface of the specific influenza virus that has been used to infect that animal. To study these antibodies, a blood (serum) sample is taken from the animal. The HI test measures how well these antibodies recognize and bind to other influenza viruses. If the ferret antibodies (resulting from exposure to the viral vaccine) recognize and bind to the influenza virus from the sick patient, this indicates that the vaccine virus is similar to the influenza virus obtained from the patient. sick. The finding has implications for how well the vaccine might work in humans.
As mentioned earlier, the influenza virus used in the HI test is obtained from samples from patients. The CDC and other WHO collaborating centers collect samples from people around the world to track which influenza viruses are infecting people and to monitor how these viruses are changing.
For the HI test, red blood cells are taken from an animal (usually a turkey or guinea pig). They are used in the HI test because the influenza virus binds to them. Normally, the red blood cells in solution will sink to the bottom of the test and form a red dot at the bottom (Figure 4A). However, when an influenza virus is added to a solution of red blood cells, the viral surface protein hemagglutinin (HA) binds to many red blood cells. When the influenza virus binds to red blood cells, the red cells form a lattice structure (Figure 4B). This keeps the red blood cells suspended in solution instead of sinking to the bottom and forming a red dot. The process by which the influenza virus binds to red blood cells to form a network structure is called hemagglutination.
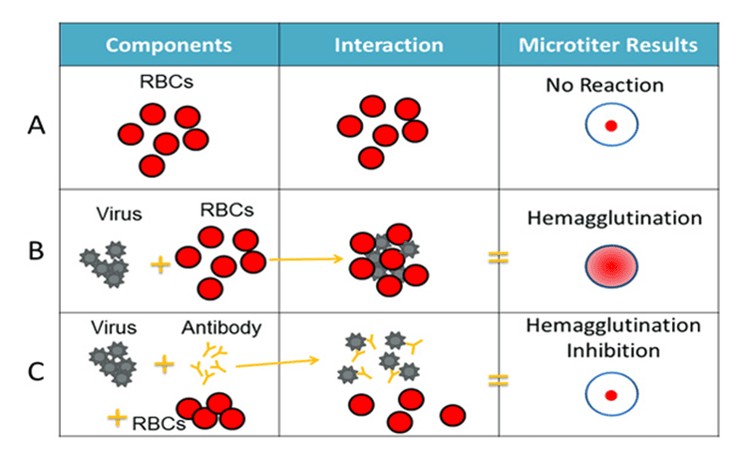
Hình 4. Các thành phần của xét nghiệm HI
The HI test involves the interaction of red blood cells (RBCs), antibodies, and the influenza virus. Row A shows that in the absence of virus, the red blood cells in the solution will sink to the bottom of the microtiter plate and look like a red dot. Row B shows that the influenza virus will bind to red blood cells when placed in the same solution. This is called hemagglutination and is represented by the formation of a lattice structure, depicted in the rightmost column according to the Microtiter results. Row C shows how antibodies are antigenically similar to a virus being tested to recognize and bind to that influenza virus. This prevents the virus and red blood cells from binding, and thus, coagulation does not occur (that is, inhibition of hemagglutination occurs instead).
When antibodies are premixed with the influenza virus followed by red blood cells, the antibodies bind to the influenza virus antigens they recognize, coating the virus so that its HA surface proteins can no longer bind. bound to erythrocytes (Figure 4C). The reaction between the antibody and the virus inhibits (that is, prevents) erythrocyte agglutination from occurring, resulting in inhibition of erythrocyte aggregation (as shown in Figure 4C). This is why the test is called the hemagglutinin suppression (HI) test. Hemagglutination (as depicted in Figure 4B) occurs when antibodies fail to recognize and bind to the influenza virus in solution, and as a result, the influenza virus binds to red blood cells in the solution, forming a conformation lattice structure. When the antibodies recognize and bind to the influenza virus in solution, this indicates that the viral vaccine (like an infected virus) has produced an immune response against the influenza virus obtained from the sick patient. . When this happens, the influenza virus under test is assumed to be antigenic in the same way that the influenza virus has produced antibodies (from ferrets).
3. Assess antigen similarity using HI . test
The HI test assesses antigenic similarity between two viruses using a scale based on antibody dilution. As mentioned previously, the HI test is performed using a microtiter plate. The microtiter plate contains rows and columns in which red blood cells, influenza virus and antibodies (developed against comparator viruses, such as viral vaccines) are mixed. The degree of dilution is marked on the top of the microtiter plate. These dilutions function as a scale for assessing antigenic interactions and immune responses. By testing the greater ability of the antibody dilution to prevent blood clotting, the scientists measured how well those antibodies recognize and bind to the influenza virus. The higher the dilution, the less antibodies are needed to prevent blood clotting, and the two viruses are more antigenically similar to each other. The highest dilution of the antibody that results in hemagglutinin inhibition is considered an HI virus. Higher HI titers are associated with greater autoantigens. Greater antigenic similarity suggests that vaccination induces an immune response against the test virus.
4. Some restrictions
Antigen characterization provides important information about whether a vaccine is used to protect against circulating influenza viruses, but there are some limitations to the antigenic characterization test method, which is described described below.
4.1. Egg adaptation Right now, most flu vaccines are made using viruses that grow in eggs. When the human influenza virus adapts to the egg, genetic changes can occur in the virus. These are called adaptive egg changes. Some egg-adaptive changes can alter the antigenic (or immunogenic) properties of the virus while others do not. Egg-adaptive changes have become a particular issue for the selection of candidate vaccine viruses for the influenza A (H3N2) virus component of influenza vaccines. Influenza A (H3N2) viruses tend to grow worse in chicken eggs than influenza A (H1N1) viruses, and they are also more susceptible to egg changes. Such changes may reduce the immune protection provided by influenza vaccines against circulating A(H3N2) viruses.

Virus cúm A (H3N2) có xu hướng phát triển trong trứng gà kém hơn các loại khác
4.2. Time from selection of the virus Vaccine to delivery of the vaccine Using current vaccine production technology, it takes approximately 6-8 months from the time the viral vaccine is selected (ie, February for influenza vaccine in the Northern Hemisphere) until the flu vaccine becomes widely available. Because influenza viruses are constantly changing, circulating influenza viruses can change during this 6-8 month period. If these genetic changes cause antigenic changes, it means that antibodies produced through vaccination may not recognize and inactivate circulating influenza viruses. New technologies to shorten the production time of flu vaccines can reduce the chance of significant antigenic changes occurring before the flu vaccine becomes available each year.
4.3. Using animals Using the HI test to assess the similarity of circulating influenza viruses to viral vaccines involves obtaining antibodies from animals (especially ferrets). Ferrets are often used as animal models for human influenza infection because they can become infected with human influenza viruses and experience similar signs and symptoms (eg, fever, sneezing, loss of appetite, etc.). When ferrets are infected with influenza viruses they often produce strong antibody responses against the influenza viruses used to infect ferrets but have little or no antibodies against other strains of influenza. Therefore, the HI test (when performed using mink serum) is the most sensitive method available for detecting antigenic differences between influenza viruses.

Chồn sương thường được sử dụng làm mô hình động vật cho nhiễm cúm ở người
Based on the recommendations of the US Centers for Disease Control and Prevention (U.S. CDC), Vinmec International General Hospital has implemented influenza vaccination in the entire hospital system across the country. for all customers 6 months and older.
Customers who choose to get vaccinated at Vinmec will be completely assured of the quality of the vaccine as well as the implementation process, because:
Children will be examined and screened by pediatricians - vaccines. Full screening of health and fitness problems, advice on preventive vaccines and injection regimens, how to monitor and care for children after vaccination before ordering vaccination according to the latest recommendations of the Ministry of Health & World Health Organization to ensure the best effectiveness and safety for children. A team of experienced and professional pediatric doctors and nurses, understand children's psychology and apply effective pain relief methods for children during the vaccination process. 100% of vaccinated children were monitored for 30 minutes after vaccination and reassessed before leaving. Underwent general supervision before, during and after vaccination at Vinmec Health System and always have an emergency team ready to coordinate with the vaccination department to handle cases of anaphylaxis, respiratory failure - circulatory arrest, ensuring Ensure timely and correct handling when incidents occur. The vaccination room is airy, with a play area, helping children feel comfortable as if they are walking and have a good mentality before and after vaccination. Vaccines are imported and stored in a modern cold storage system, with a cold chain that meets GSP standards, keeping vaccines in the best conditions to ensure quality. Parents will receive a reminder message before the vaccination date and their child's vaccination information will be synchronized with the national immunization information system. Vinmec International General Hospital is currently providing a Package Immunization Program with a variety of vaccines for different audiences, from infants, young children, adults, and women before and during pregnancy pregnant.
If you have a need for consultation and examination at Vinmec Hospitals under the nationwide health system, please book an appointment on the website for service.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
Reference source: cdc.gov