This is an automatically translated article.
Attenuvax is also known commonly as the live attenuated measles vaccine for intradermal administration. Attenuvax is recommended for use in children from birth to 12 months of age. So to find out specifically what Attenuvax does? How to use through the article below.
1. The effect of the drug Attenuvax
Vaccine Virus Vaccine Live is an immunogenic agent used to prevent infection by the measles virus. It works by causing your body to create its own protection (antibodies) against the virus. This vaccine does not protect you against German measles (Rubella). A separate vaccination is needed for this type of measles.
2. How to use Attenuvax?
2.1. How to take Attenuvax Measles (also known as cough measles, hard measles, morbilli, red measles, rubella, and ten-day measles) is an infection that spreads easily from person to person. Measles infection can lead to serious problems, such as pneumonia, ear infections, sinus problems, seizures, brain damage, and possibly death. The risk of serious complications and death is higher for adults and infants than for children and adolescents.
Measles vaccination is recommended for everyone 12 to 15 months of age and older. In addition, there may be special reasons why children aged 6 months to 12 months may also need the measles vaccine. However, vaccination against measles is not generally recommended for infants up to 12 months of age, unless the risk of measles infection is high. This is because the antibodies they receive from their mother before birth can affect the effectiveness of the vaccine. Children who were vaccinated against measles before 12 months of age should be vaccinated twice more.
In addition, if you have had two doses of measles vaccine starting on or after your first birthday and have medical records to prove it, if you were previously diagnosed with measles by your doctor , or if you have had a blood test showing immunity to measles.
2.2. Dosage of Attenuvax Each patient will have a different dose of Attenuvax. Therefore, it is important to follow your doctor's prescription or the directions on the label. The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and how long you take the medicine depend on the medical problem for which you are using the medicine.
For the injectable form, for measles prevention, adults and children 12 months of age and older, 1 dose subcutaneously, followed by a second dose at least one month later.
3. Contraindications of Attenuvax
Children under 6 months of age History of severe allergic reaction (eg, anaphylaxis) to any component of the vaccine or after a previous dose of influenza vaccine.
4. Notes when using Attenuvax
When deciding to use a vaccine, the risks of vaccination must be weighed against the benefits it will do. This is a decision that you and your doctor will make. For this vaccine, consider the following:Allergies Tell your doctor if you have ever had any unusual or allergic reaction to Attenuvax or any other medicine. Also tell your healthcare professional if you have any other types of allergies, such as to foods, dyes, preservatives, or animals. For over-the-counter products, read the label or package ingredients carefully.
Pediatrics Measles vaccine is not generally recommended for infants up to 12 months of age. In special circumstances, such as children traveling outside of the United States or children living in high-risk areas, the measles vaccine can be given to children as young as 6 months of age.
Breastfeeding Women Studies in women have shown minimal risk to the infant when used during breast-feeding and without pregnancy for 3 months after measles vaccination without control. Check with your doctor first.
Talk to your doctor that you have received this vaccine: If you received a Tuberculin skin test within 4 to 6 weeks of receiving this vaccine. Test results may be affected by this vaccine.
If you received this vaccine within 2 weeks before or 3 to 11 months after receiving a transfusion of blood or other blood products.
If you received this vaccine 2 weeks before or 3 to 11 months after receiving gamma globulin or other immunoglobulins.
5. Attenuvax side effects
Along with its necessary effects, a drug can cause some unwanted effects. Although, not all of these side effects are possible, but if they do, check with your doctor right away:
Difficulty breathing or swallowing Itching, especially red feet or hands skin, especially around the ears Swelling of the eyes, face, or inside of the nose Unusual tiredness or weakness (sudden and severe) Check with your doctor as soon as possible if any of the following side effects occur:
More common:
Fever above 103°F (39.4°C) Rare:
Bruises or purple spots on the skin Confusion Double vision Headache (severe or continuing) Irritability Neck stiffness Swelling, blistering or pain at the injection site Swelling of glands in the neck Vomiting Some side effects can occur that usually do not require medical attention. These side effects may go away during treatment as your body adjusts to the medication. In addition, your healthcare professional may be able to tell you about ways to prevent or reduce some of these side effects. Check with your healthcare professional if any of the following side effects continue or cause discomfort or if you have any questions about them:
More common:
Burning or stinging at the site injection Fever of 100°F (37.7°C) or less Less common:
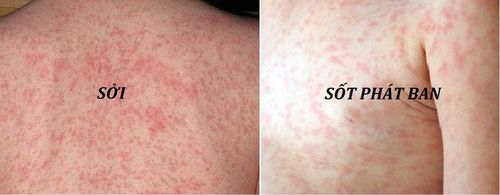
Fever of 100 to 103°F (37.7 to 39.4°C) Itching, swelling, redness, pain, or hard lump at injection site Skin rash A fever or skin rash may occur 5 to 12 days after vaccination and usually lasts several days. Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional. and seek emergency medical attention or call 911.
6. Storage of Attenuvax
Store lyophilized vaccine vials at 2°C to 8°C or lower. The diluent may be stored with the lyophilized vaccine powder or stored separately at room temperature. Do not freeze solvents. The vaccine should be administered as soon as possible after reconstitution. The reconstituted vaccine can only be stored in the vial in the dark between 2°C and 8°C and up to 8 hours. After this time, the vaccine must be discarded.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References: rxlist.com, drugs.com, holevn.org