This is an automatically translated article.
Posted by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General HospitalVirulence factors of H. pylori and its role in systemic diseases. To survive in an unfavorable and hyperactive stomach, H. pylori synthesizes a number of virulence factors that both improve conditions for living in an acidic environment and damage the gastric mucosa. .
1. Biological properties of H.pylori
In their work, Kao et al. noted that when entering the host stomach, H. pylori uses the activity of urease to neutralize hydrochloric acid, which is one of the protective and effective factors. markedly antibacterial. The regulation of urease synthesis is encoded by several genes known as urease gene clusters. This genome consists of catalytic units (urea A/B), an acid-differentiated urea channel (ureaI) and sub-assembly proteins (urea EH).
2. The synthesis of urease depends on the pH around the bacteria.
Of interest is that urease synthesis is pH dependent around the bacteria: The ureaI channels are tightly closed at pH 7.0 and fully open at pH 5.0. That is, when the external conditions change, namely when the acidity in the gastric lumen increases, H. pylori releases the enzyme urease which hydrolyzes urea into CO2 and ammonia (NH3), which then binds to water and form unstable ammonium hydroxide. This series of biochemical reactions leads to moderate alkylation. This acid-barrier crossing mechanism is critically important for bacterial survival along with its helical shape, smooth cell wall, and helical motion. Schoep et al demonstrated that urease-negative bacteria were not able to penetrate the gastric mucosa of piglets by gnotobiotics. Another important virulence factor promoting the spread of bacteria to the gastric epithelium is the mobility of this bacterium due to the presence of 4-7 motile flagellates. The flagellate is a complex organelle composed of several types of protein subunits and includes a basal body, hooks, and filaments. There are several types of flagellate peristalsis: “swimming peristalsis”, “spreading peristalsis”, and “herd peristalsis”. Together with urease activity, flagellate peristalsis has been shown to be a necessary factor for gastric mucosal penetration. There are also studies that demonstrate the primary importance of flagellates in the formation of microbial biofilms on the surface of gastric mucosa.3. Bacterial adhesion is an important stage of mucosal invasion
Bacterial adhesion is an important stage of the invasion process, deciding the entire existence of H. pylori in the stomach. The literature describes adhesion molecules (outer membrane proteins), such as blood antigen-binding protein A, sialic acid-binding binder, neutrophil activation protein, heat shock protein (Hsp) 60, antigen-binding proteins (AlpA and AlpB), the H. pylori outer membrane protein, and the LacDiNAc-binding binder. Among the main factors of invasion, virulence factors that have a direct damaging effect on the gastric mucosal epithelium can be separated; this is cagA, γ-glutamine transferase, which requires high temperature A and vacuum cytoxin A (vacA).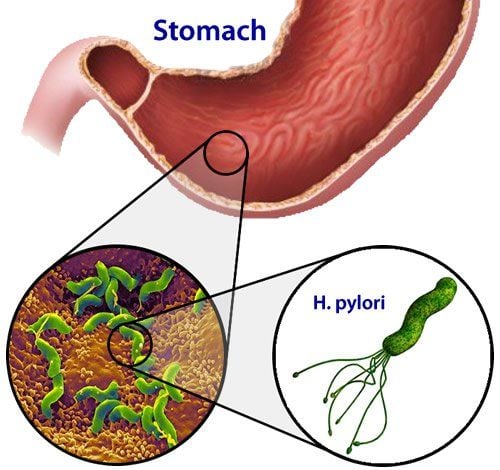
CagA is a highly antigenic protein with a molecular weight of 120-145 kDa. The site of the gene responsible for cagA synthesis and the type IV secretion system (T4SS) was named cag pathogenic island. CagA acts intracellularly through epithelial cells via T4SS: The latter forms a syringe-like ciliary structure through which the cagA molecule enters host epithelial cells. After translocation into the cell, cagA undergoes phosphorylation on the inner surface of the epithelial cell cytoplasmic membrane and thus acquires biochemical activity. Experimentally it has been found that mice competent to phosphorylate cagA have developed cancers, such as gastrointestinal adenocarcinoma, myeloid leukemia, and B-cell lymphoma. Furthermore, these pathogenic processes were not observed in antiphosphorylated forms. The main pathogenic effect of cagA is to enhance the mitotic activity of gastric epithelial cells, which contributes to malignancy if the bacteria persist for a long time.
VacA protein VacA is a protein with a molecular weight of 88 kDa, consisting of two subunits (p33 and p55) and has a wide range of disease genetic effects. Following protein internalization, large pores are formed in the cytoplasmic membrane of gastric epithelial cells, which makes the cell more susceptible to the action of bacterial urease. Beyond that point, the initiation of a series of biochemical reactions promotes the accumulation of large vacuoles inside the cell, leading to functional impairment of epithelial cells. Through the intracellular transport system, vacA can enter mitochondria, where it disrupts the integrity of the mitochondrial inner membrane. This reduces the mitochondrial transmembrane potential (∆Ψm) with subsequent release of cytochrome C and activation of the proapoptotic factor-binding protein X-binding Bcl-2. There are data to suggest that vacA is associated with the avoidance of a marked immune response to infectious agent entry. This is achieved by both reducing T-lymphocyte activation in the stroma and by disrupting autophagy.
4. Immune response to H. pylori invasion
Once entering the human body, the H. pylori bacteria is continuously controlled by the immune system. An inflammatory response is known to be a marker for a developing immune response. In the persistence of H. pylori, the focus of inflammation mainly affects gastric epithelial cells; in this case, the inflammatory response involves neutrophils, lymphocytes, macrophages, and dendritic cells (DCs), which migrate to the site of infection through the circulatory system Exposure of DCs with H. pylori antigenic determinants leads to autoendocrine activation of the immature CD4+ T cell group, followed by their differentiation into T-helper(Th)1 lymphocytes through through the production of interleukin (IL)-12. There are Th1 lymphocytes that are the main inflammatory cells for H. pylori entry. In addition, proinflammatory cytokines, such as IL-1, IL-6, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ, are involved in the immune response
Experiments have demonstrated that vacA can exert an immunosuppressive effect on cells of the immune system, by way of inhibiting IL-23 production by DCs. Furthermore, H. pylori HspB has the ability to inhibit the proliferation of mitogen-stimulated T cells, by enhancing the inhibitory effect of regulatory T cells (Tregs) in both immune responses. Innate and adaptive H. pylori interaction with Th2 lymphocytes is unclear
Due to the activation of the Th1-cell component of the immune system, the Th2-cell pathway for lymphocyte differentiation has not yet been established. Maturity is not thought to play a significant role in the immune response. At the same time, it has been shown that immunoglobulin G (IgG) level is a reliable indicator of H. pylori persistence. In infected individuals, the Th2 response induces IgG1 while the Th1 response contributes to a significant increase in the overall level of IgG2 through the production of IL-2 and IFN-γ. The IgG2 titer is higher than the IgG1 titer in patients with H. pylori infection, especially in those with ulcerative disease. Thus, the persistence of H. pylori in the human body is accompanied by a marked immune response to bacterial invasion, which is subsequently replaced by a tolerable immune response. This fact may suggest that the relationship between H. pylori and the host is symbiotic.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References Reshetnyak VI, Burmistrov AI, Maev IV. Helicobacter pylori : Commensal, symbiont or pathogen? . World J Gastroenterol 2021; 27(7): 545-560 [PMID: 33642828 DOI: 10.3748/wjg.v27.i7.545]