This is an automatically translated article.
Post by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General Hospital
Knowledge about coronavirus disease 2019 (COVID-19) is growing rapidly. Although patients with inflammatory bowel disease do not appear to be at high risk for COVID-19, the potential impact of immunosuppressive therapies on inflammatory bowel disease patients infected with coronavirus 2 acute respiratory syndrome serious concern for clinicians and patients.
Several recommendations and guidelines have recently been published, including the reorganization of endoscopic and gastrointestinal services needed for these patients, the growing role of telemedicine, and the importance of telemedicine. importance of addressing aspects of mental health in this context.
1. How to manage inflammatory bowel disease during the COVID-19 pandemic
Management of patients presenting to the outpatient clinic with inflammatory bowel disease in remission in the setting of asymptomatic SARS-CoV-2 infection or confirmed or suspected COVID-19 without systemic inflammatory syndrome During a pandemic As COVID-19 expands, more and more testing for SARS-CoV-2 is underway, including asymptomatic COVID-19 exposure index cases. As a result, the situation where an individual tests positive for the virus but remains asymptomatic will become more and more frequent.
The International Organization for the Study of Inflammatory Bowel Disease (IOIBD) recommends that for patients with reassured inflammatory bowel disease with asymptomatic SARS-CoV-2 infection - prompt withdrawal of prednisone or dose reduction to <20 mg/day or switch to budesonide or budesonide MMX as appropriate. Immunomodulators such as thiopurines, methotrexate, and tofacitinib (or other Janus kinase inhibitors) should be temporarily withheld for 2 weeks while monitoring for the emergence of COVID-19 symptoms. Similarly, the administration of biologics, including anti-TNF agents, vedolizumab and ustekinumab, should be postponed for 2 weeks if the dose is exhausted, even when it is recognized that the half-lives of these biologicals are relatively long, because that the immunosuppressive effects of these drugs will persist for several more weeks even though these agents have withdrawn. In contrast, nonimmune-based anti-inflammatory therapies such as aminosalicylates, antibiotics, budesonide or rectal therapy can be continued. However, for patients with inflammatory bowel disease who have had close contact with an individual with proven or suspected COVID-19 they should self-isolate and follow local recommendations from medical management. In this situation, the European Crohn's and Colitis Organization (ECCO) experts recommend that it is not necessary to keep biologics or immunomodulators based solely on exposure
For the patient Outpatient clinic treatment with silent enterocolitis but with confirmed or suspected COVID-19, the same drug management approach as is applied to patients with asymptomatic SARS-CoV-2 infection evidence . Experts from the American Gastroenterological Association (AGA) and the International Organization for the Study of Inflammatory Bowel Disease recommend that budesonide, aminosalycilate, antibiotics, and topical therapy should be maintained while systemic corticosteroids should be avoided. prednisone) and withdraw rapidly, if possible. Likewise, immunomodulators, Janus kinase inhibitors, and biologics should be withheld until symptoms disappear, usually for 2 weeks during the acute illness
Management of patients with active inflammatory bowel disease undergoing outpatient follow-up in the setting of asymptomatic SARS-CoV-2 infection or confirmed or suspected COVID-19 without systemic inflammatory syndrome Present, In the COVID-19 era, if an inflammatory bowel disease patient presents with an obvious flare-up, it is always important to question the presence of symptoms suggestive of COVID-19, such as fever, cough, anemia, or dyspnea, as gastrointestinal symptoms including diarrhea, nausea, vomiting, and abdominal pain have been reported in 2%-33% of patients at initial COVID-19 presentation. Furthermore, in some cases, these gastrointestinal symptoms may be the only clinical feature of COVID-19. This context presents a clinical challenge, combined with the routine detection of significantly increased serum inflammatory biomarkers in COVID-19 patients.
When an inflammatory bowel disease patient presents with diarrhea, suspecting whether this is secondary to a disease outbreak or COVID-19, a wait-and-see approach for the next 5-7 days is a strategy. As a reasonable strategy, once diarrhea due to COVID-19 is mostly mild and self-limited, usually with a mean duration of 5 days (range, 1 day to 14 days) and a mean frequency of 4 episodes. defecate every day. In addition, monitoring using interval assessment of fecal calprotectin (FC) may be helpful, as FC levels are often transient and mildly elevated in patients with diarrhea due to COVID-19. In contrast, in active inflammatory bowel disease, persistent and significant high FC levels are often seen. In any event, in the current era of COVID-19, the general expert consensus from ECCO recommends that all patients with suspected inflammatory bowel disease be tested to rule out COVID-19, preferably especially with oropharyngeal and nasopharynx reverse transcriptase polymerase chain reaction (RT-PCR) testing when the first symptoms appear In cases where, after initial evaluation, diagnostic doubts remain, then Computed tomography (CT) of the chest, transabdominal imaging and, to a lesser extent, endoscopic evaluation with ileocolonoscopy may allow identification of the true cause of diarrhea. Another question that remains unknown is whether SARS-CoV-2 can cause outbreaks or de novo inflammatory bowel disease. Furthermore, in patients with clearly active inflammatory bowel disease (especially inflammatory bowel disease of the colon), it is important to know to rule out intestinal superinfection, primarily due to Clostridioides difficile (C. difficile) and cytomegalovirus (CMV), assessment of adherence, and biomonitoring drug implementation.
If gastrointestinal symptoms (including diarrhea) are not due to COVID-19 and other causes of inflammatory bowel disease are excluded, such as intestinal superinfection, use of non-steroidal anti-inflammatory drugs and non-adherence, medication management for IBD will depend on the balance between the severity of the inflammatory bowel disease flare-up and that of COVID-19. For mild outbreaks in outpatients with asymptomatic SARS-CoV-2 infection or with mild to moderate COVID-19 without systemic inflammatory syndrome (SHS), recommend report a reduction in prednisone or equivalent to a dose <20mg/day. Discontinue the drug completely if possible, balancing the possibility of adrenal insufficiency that may occur with chronic corticosteroid therapy. Another option that may be considered in patients taking systemic steroids is conversion to oral budesonide or MMX budesonide at an appropriate dose, provided the patient is in an appropriate clinical condition (eg. , Crohn's disease or mild to moderate ulcerative colitis). Furthermore, it is recommended to discontinue or avoid initiating immunomodulatory drugs, tofacitinib (or other Janus kinase inhibitors), and biologics for at least 2 weeks during viral illness, while budesonide, aminosalycilates, antibiotics and topical therapy may be initiated or maintained as needed. Treatment of moderate to severe flare-ups of inflammatory bowel disease in outpatients with COVID-19 no SHS may include continuation of current biologic therapy for inflammatory bowel disease with optimization to salvage remission or initiation of a new biologic agent if needed, preferably in monotherapy materials and with subcutaneous biologics to reduce the risk of exposure to SARS-CoV-2 in infusion units.
In this clinical setting, if glucocorticosteroids are considered essential, the dose of prednisone (or equivalent) should be ≤ 40 mg/day of limited duration of use, if possible. In addition, treatment with immunomodulators or tofacitinib should be stopped or avoided. If COVID-19 progresses with significant lung damage and requires hospitalization, it is advisable to consult an infectious disease specialist so that COVID-19 can be treated with antiviral anticytokine therapy. or experiment can be interesting. In Tables 1-3, the authors present an approach to drug management of inflammatory bowel disease in outpatient clinic patients infected with SARS-CoV-2 with or without COVID-19.
Management of patients presenting to the outpatient clinic with inflammatory bowel disease who are well in the case of coronavirus 2 asymptomatic acute respiratory syndrome or confirmed or suspected coronavirus disease 2019
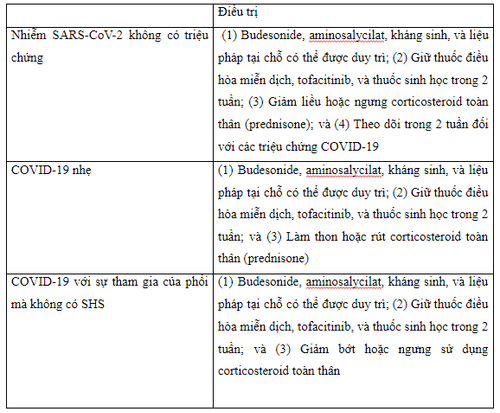
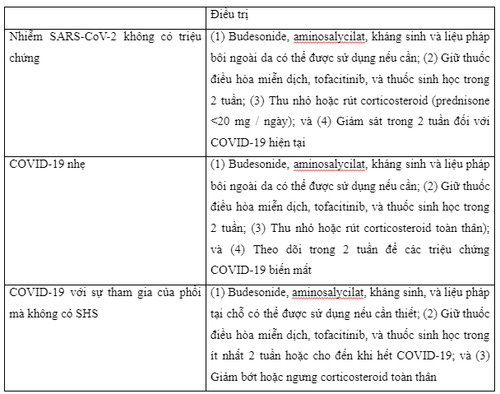
Management of outpatient visits with moderate to severe active inflammatory bowel disease in cases of coronavirus 2 asymptomatic acute respiratory syndrome or confirmed or suspected coronavirus disease 2019
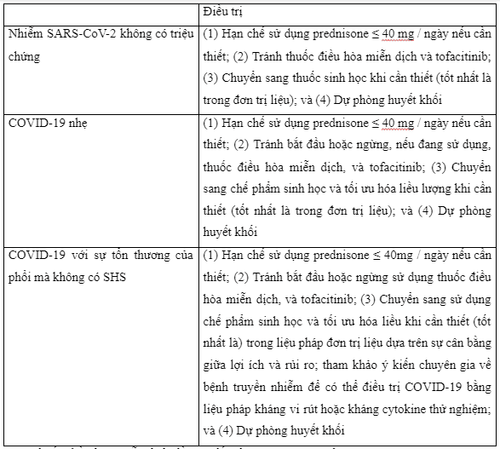
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References Chebli JMF, Queiroz NSF, Damião AOMC, Chebli LA, Costa MHM, Parra RS. How to manage inflammatory bowel disease during the COVID-19 pandemic: A guide for the practicing clinician. World J Gastroenterol 2021; 27(11): 1022-1042 [PMID: 33776370 DOI: 10.3748/wjg.v27.i11.1022]