This is an automatically translated article.
Posted by Master, Doctor Mai Vien Phuong - Gastrointestinal Endoscopy - Department of Medical Examination & Internal Medicine - Vinmec Central Park International HospitalThe initial development of AI was based on creating an algorithm using highly accurate data sets, coded in an independently organized way by a team of experts. This process is called “foundational truth” establishment, where the computer is prepared to distinguish between “abnormal” and “normal” tissue.
1. What is AI (Artificial intelligence)?
CAD systems using advanced AI techniques represent an innovative technology that has the potential to lead to a paradigm shift in the field of diagnostic colonoscopy. Since endoscopy often involves computer vision technology, this technique allows computers to "see" and decode visual content. Through machine learning and, more recently, learning and memory processes, AI systems can be set up to recognize “normal” features by connecting the gold standard to the right image.Deep learning is a method of computer learning, recently using deep neural networks, that automatically extracts specific features from data without human effort if some are available. very large number of learning samples.
The initial development of AI was based on creating an algorithm using highly accurate data sets, coded in an independently organized way by a team of experts. This process is called “foundational truth” establishment, where the computer is prepared to distinguish between “abnormal” and “normal” tissue. Once an algorithm is created, complex neural networks use vast data sets to allow the algorithm to become more skilled. This intelligence is extendable to algorithms and it can learn by developing its own rules and classifiers.
AI is expected to have at least 2 major roles in colonoscopy practice: Colon polyp detection (CADe) and polyp characterization (CADx). CADe has the potential to reduce polyp missed rates, contributing to improved detection of adenomas, while CADx can improve the accuracy of optical diagnosis of colorectal polyps, leading to a reduction in unnecessary removal of lesions. noncancerous lesions, potential for performing resection and resection, and appropriate strategies and application of advanced resection techniques.
2. What are colon polyps?
Colon polyps are small masses of cells that form on the lining of the colon (large intestine). Most colon polyps are harmless, but over time, some colon polyps can develop into colon cancer (CRC), which is fatal when found in its late stages.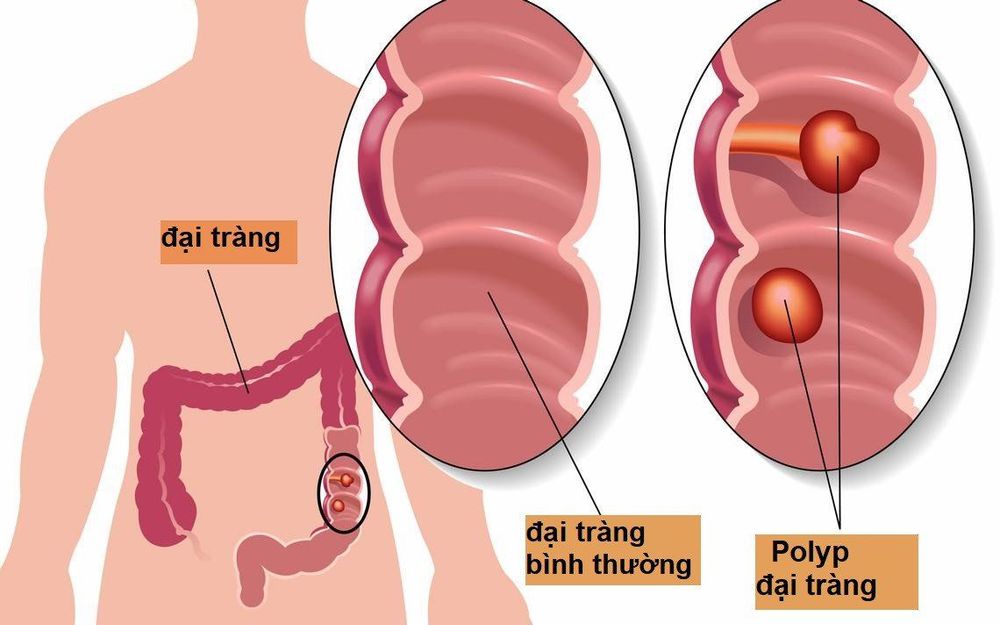
3. How can AI artificial intelligence help assess colon polyp characteristics?
To reduce CRC-associated morbidity and mortality, there is also a major factor other than lesions that needs to be removed: Accuracy of characterization and classification of small polyps (<5mm) by trained endoscopists are reported to be < 80%. Furthermore, resection of noncancerous/hyperplastic polyps, which almost never progress to malignancy, presents a financial burden, an unnecessary intervention performed on a healthy tissue, and a excision and/and observation of these unrelated polyps both represent unnecessary waste of time.Recently, CADx AI system for colon polyp characterization and diagnosis, optical biopsy and histological prediction classification has been developed by many groups using computational analysis to predict polyp histology. . CADx are applications of AI where the system helps the physician interpret the content of a medical image by labeling the image itself. Thus, AI systems support real-time optical biopsies by providing classification of colon polyps into a category (e.g. adenoma, hyperplasia, etc.) that is CADx. Neural Convolutions networks that can perform such a task are called image classifiers and research in this area has been very active in the last 5 years.
Both the accuracy, sensitivity, specificity, positive predictive value and negative predictive value (NPV) as well as the feasibility of these systems have been described recently: Aihara et al. The first published a prospective study of 32 patients with 102 colorectal lesions, using CAD to differentiate cancer from noncancerous lesions using the CADx system allowing for color analysis. "real-time" of colorectal lesions as applied to automated fluoroscopy to perform color-sampling aims to reduce unnecessary treatments for noncancerous lesions ; All lesions larger than 5 mm were resected endoscopically and lesions smaller than 5 mm were biopsied. They concluded that lesions with a green/red ratio less than 1.01 were cancerous while those above the cut were considered noncancerous, with sensitivity, specificity, and NPV of 94, respectively. 2%, 88.9% and 85.2%.
Kuiper et al used a system called WavSTAT for real-time optical diagnosis based on laser-induced auto-fluorescence spectroscopy in 87 patients with 207 small (<10 mm) colorectal lesions. ). During endoscopy, the endoscopist attempts to distinguish adenomas from non-adenomas with high confidence. Then, all lesions were systematically analyzed. Histopathology was used as the reference standard.
Accuracy and NPV of WavSTAT were 74.4% and 73.5% for WavSTAT alone, respectively, while they were 79.2% and 73.9% respectively when combining WavSTAT with resolution endoscopy The study concluded that it did not meet the performance thresholds of the American Society of Gastroenterology (ASGE) for the evaluation of small and small lesions. Rath et al used the WavSTAT4 optical biopsy clamp system designed by Spectrascience Inc, San Diego, California, USA for histological prediction by laser-induced auto-fluorescence spectroscopy in 27 patients. multiplied by 137 small polyps (≤ 5 mm). Accuracy was 84.7% along with sensitivity of 81.8%, specificity of 85.2% and NPV of 96.1%. They conclude that this new WavSTAT4 system has the potential to meet the ASGE thresholds for a “resend and discard” strategy.
Kominami et al used a narrow band image magnifying colonoscopy (NBI) system for a CAD real-time image recognition system with support vector machine output of colorectal polyp histology over 41 patient with 118 colorectal lesions. The concordance between the endoscopist and CAD was 97.5%. Accuracy, sensitivity, specificity, positive predictive value and NPV were 93.2%, 93%, 93.3%, 93% and 93.3%, respectively, concluding that this CAD system could meet the recommendations of the ASGE Valuable Endoscopic Conservation and Incorporation committee for resend and discard strategies.
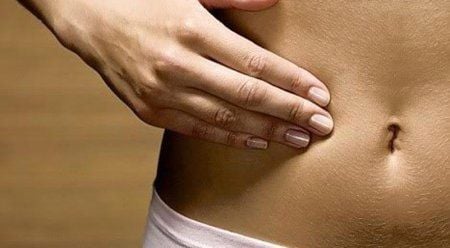
Table 2: Computer-aided detection and diagnosis systems that can achieve conservative thresholds and incorporate valuable endoscopic improvements for small, noncancerous rectal polyps.
| Tham khảo | Mục tiêu | Hệ thống | Hiệu suất | Năm | Quốc gia |
| Aihara và cộng sự | Tất cả các polyp ( n = 102) | AFE | Độ nhạy: 94,2% Độ đặc hiệu: 88,9% NPV: 85,2% | 2013 | Nhật Bản |
| Kuiper và cộng sự [ 36 ] | Polyp <10 mm ( n = 207) | WAVSTAT, WAVSTAT + HRE | Độ chính xác: 74,4% Độ chính xác + HRE: 79,2% NPV: 73,5% NPV + HRE: 73,9% | 2015 | Nước Hà Lan |
| Rath và cộng sự | Polyp ≤ 5 mm ( n = 137) | WAVSTAT4 + CUỘC SỐNG | Độ chính xác: 84,7% Độ nhạy: 81,8% Độ đặc hiệu: 85,2% NPV: 96,1% | 2016 | Nước Đức |
| Kominami và cộng sự | Tất cả các polyp ( n = 118) | NBI | Độ phù hợp: 97,5% Độ chính xác: 93,2% Độ nhạy: 93% Độ đặc hiệu: 93,3% PPV: 93% NPV: 93,3% | 2016 | Nhật Bản |
| Mori và cộng sự | Polyp ≤ 5 mm ( n = 466) | EC-NBI-CAD EC-MB-CAD | Độ chính xác: 98,1% NPV EC-NBI-CAD: 96,5% NPV EC-MB-CAD: 96,4% | 2018 | Nhật Bản |
4. Future Prospects of AI
These technologies are evolving rapidly and are expected to also lead to a reduction in the costs associated with unnecessary colonic polypectomy and perhaps, in the future, to completely replace medical screening. management of the excised tissue, thus specifying histological features and the possibility of associated malignancy, and monitoring for potential consequences immediately, during colonoscopy. In addition, most of the proposed AIs aim to create a system that can write automated endoscopy reports. The main advantage of developing this aspect would be to increase the accuracy of the report itself, which today can still be influenced by the subjective biases of different endoscopists both in describing the position and location and characteristics of the polyp, such as the size or appearance of the polyp. In this way, endoscopists are expecting that this technology will help standardize real-time lesion characteristics and also endoscopic reports. Automated reporting will also help the endoscopist avoid wasting time in report writing and thus can also increase the rate of endoscopic examinations and interventions performed by the gastrointestinal endoscopy unit.
5. Conclusion
AI is an emerging technology whose application is rapidly increasing in many medical fields. Several applications of AI in gastroenterology are showing promising results, especially in the treatment of gastrointestinal cancers. Among these, techniques that can increase ADR will play an important role in reducing the incidence of CRC and its associated mortality from undetected or misclassified space cancers. Moreover, it is also expected to significantly reduce costs. In addition, the support of the machine will help reduce the examination time and thus optimize the endoscopic schedule.Furthermore, indication for polypectomy, supported by good sensitivity and specificity, will lead to a reduction in the direct cost of unnecessary polyps. Furthermore, higher diagnostic accuracy in identifying precancerous and cancerous lesions will lead to a reduction in the secondary costs of prevented cancers.
Despite promising results, AI techniques to detect and identify colorectal lesions still require further investigation in the near future. In particular, since most of the results come from trials conducted in highly specialized centres, presenting a limitation on the generalizability of the results, they must also be validated in clinical practice.
Finally, the integration of AI in a fundamentally human medical environment must be considered: AI was not conceived, nor is it now, never, to replace the endoscopist, on the contrary, it seems to be an extremely useful tool to be used since the endoscopist himself, with his abilities and skills, is the only one who can process and interpret all the AI information to make informed decisions. on patient management.
Currently, screening for gastrointestinal cancer is a scientific and effective measure for early detection of gastrointestinal cancer (esophageal cancer, stomach cancer, colon cancer) and giving a treatment plan. best treatment. Currently, Vinmec International General Hospital has a package of screening and early detection of cancers of the gastrointestinal tract (esophagus - stomach - colon) combined with clinical and paraclinical examination to bring the most accurate results. maybe.

Gastroenterology specialist examination with an oncologist (by appointment). Gastroscopy and colonoscopy with an NBI endoscope with anesthesia. Peripheral blood count (laser counter). Automated prothrombin time test. Automated thrombin time test. Activated Partial Thromboplastin Time (APTT) test using an automated machine. General abdominal ultrasound
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
ReferencesEmanuele Sinagra, Matteo Badalamenti, et al., Use of artificial intelligence in improving adenoma detection rate during colonoscopy: Might both endoscopists and pathologists be further helped. World J Gastroenterol. Oct 21, 2020; 26(39): 5911-5918 Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] Maida M, Morreale G, Sinagra E, Ianiro G, Margherita V, Cirrone Cipolla A, Camilleri S. Quality measures improving endoscopic screening of colorectal cancer: a review of the literature. Expert Rev Anticancer Ther. 2019;19:223-235. [PubMed] [DOI]