This is an automatically translated article.
The article is written by Master. Doctor Doan Trung Hiep, Head of the Department of Radiation Therapy, Radiation Therapy Center, Vinmec Times City International HospitalOn October 5, 2020, a three-person research team (Harvey Alter, Charles Rice from USA, Michael Houghton from UK, now living in Canada) was awarded the Nobel Prize in Biomedical Sciences for the discovery of Hepatitis C Virus, The discovery of HCV saves millions of lives on earth every year, increases screening standards in blood donation and blood transfusion, helps develop the pharmaceutical industry to find anti-HCV drugs, and reduces the rate of HCV-related liver cancer.
1.Introduction
Harvey J.Alter, doctor of the US National Institutes of Health (NIH).
Michael Houghton, PhD, University of Alberta, Alberta, Canada, born in the UK.
Charles M.Rice, Rockefeller University, New York, USA.
In 1979, people discovered HAV.
In 1965, HBV was discovered.
However, there is still an unexplained hematogenous infection-associated hepatitis. And in 1989, a group of 3 scientists discovered HCV.
Secretary of the Nobel Committee for Biomedical Sciences, Dr. Perlmann said: “I woke them up, I made a few phone calls to Alter and Rice and they were amazed at this joy and were speechless, It's really fun talking to them."
Rice, born in Sacramento, the capital of California, studied and worked at the University of Washington in Saint Luis before becoming director of the Hepatitis C Research Center at Rockefeller University in 2001.
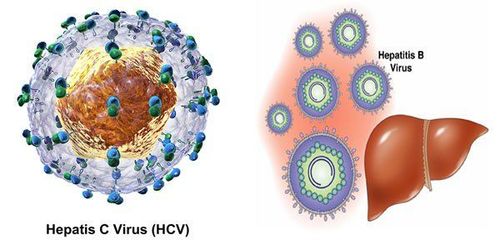
Alter, studied viral hepatitis before the discovery of HCV, in 1960 he jointly discovered an Australian antigen - a decisive point in the research to detect HBV.
Houghton, completed his doctoral thesis in biochemistry at King's College London in 1977 and moved to California in 1982 to settle at Chiron company, then in 2010 he moved to Alberta, Canada, as a director. Director of the Center for Applied Virology at the University of Alberta.
The Nobel Prize consists of a medal and 1.1 million US dollars to be divided equally among the three scientists.
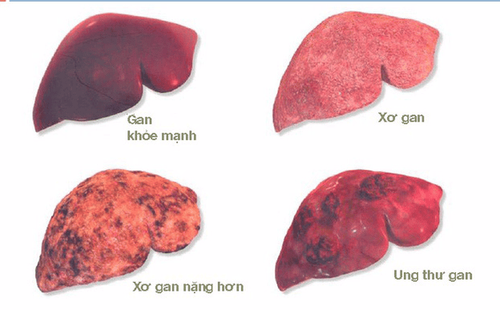
2.HCV is a causative agent of liver cancer
According to WHO, globally 71 million people are infected with HCV, HCV causes 400,000 deaths/year due to cirrhosis and liver cancer
HCV is a direct carcinogen, although this virus does not integrate directly into genome of infected human hepatocytes, which through the activities of “Intracellular communication”, proliferative activity, “programmed death”... after a period of infection if not well controlled causes cirrhosis and liver cancer.
The risk of liver cancer for HCV-infected people with cirrhosis is 3-5%/year.
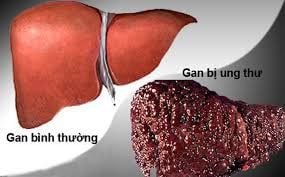
What risk factors promote cancer in people infected with hepatitis B and C.
The biggest risk factors are: simultaneous infection with hepatitis B and C viruses; HIV infection, liver disease caused by alcohol abuse, age (with increasing age, the risk increases with age), men are at higher risk than women.
Some co-morbidities such as obesity and diabetes increase the risk of liver cancer in people infected with HCV types 1c and 3.
Particularly, cancer risk factors are associated with complicated hepatitis B infection. more than: include all patient-related factors similar to hepatitis B risk factors (simultaneous infection with hepatitis B and C viruses; HIV infection, alcohol abuse liver disease, age (the older the risk increases with age), men have a higher risk than women); family history of HBV infection, hepatitis C and D infection, alpha-toxin exposure, poor control of HBV such as persistently elevated liver enzymes (with persistent hepatocellular damage), HBeAg positivity, measure high HBV-DNA load (over 2000 IU), HBV infection type D, B as well as primer mutations.
3. Should liver cancer screening be done for everyone infected/carried with HCV, HBV?
The risk of liver cancer for people with HCV infection is 1.5%/year, for people with HBV infection 0.2%/year.
3.1 The following groups of people should be screened for liver cancer
HBV infection group
All HBV infected patients had cirrhosis. African Americans, African Americans, Asian men 40 years of age or older, Asian women 50 years of age or older. HCV-infected group
All HCV-infected persons with signs of cirrhosis or suspected cirrhosis should be screened for liver cancer. Screening facilities and frequency of liver cancer screening
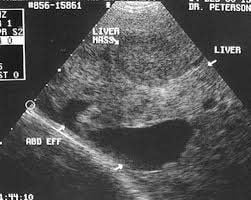
AFP test and liver ultrasound every 6 months are widely recommended for the population as outlined above. Vinmec International General Hospital has standard to advanced Hepatobiliary Screening Packages, which help detect Hepatitis Virus at an early stage even when there are no symptoms. In addition, the comprehensive hepatobiliary screening package helps customers:
Assess the liver's ability to work through liver enzyme tests; Evaluation of bile function; vascular nutrition; Early screening for liver cancer; Perform tests such as Total blood cell analysis, blood clotting ability, screening for hepatitis B, C. Assessment of hepatobiliary status through ultrasound images and diseases that are at risk of affecting liver disease/ worsen liver disease. In-depth analysis of parameters to evaluate hepatobiliary function through laboratory and subclinical; the risk of affecting the liver and early screening for hepatobiliary cancer.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
4. Does the treatment and good management of HCV and HBV reduce the risk of liver cancer?
4.1 For HBV infected group Antiretroviral drugs such as Lamivudine and adefovir reduce the risk of liver cancer by 51% in people infected with HBV.
Currently, tenofovir and entecavir (both of which are readily available and widely used in Vietnam) inhibit 95% of HBV and reduce viral resistance and are used as first-line agents in HBV management.
In terms of the risk of liver cancer progression of the HBV-infected group, treatment with tenofovir and entecavir significantly reduces the risk of liver cancer if the infected person has not had cirrhosis at the time of initiation of treatment, treatment duration 6 five; In the group of people infected with HBV who already had cirrhosis, tenofovir did not reduce the risk of liver cancer.
Therefore, use prevention of HBV infection (safe blood transfusion, safe sex, prevention of mother-child transmission, use of safe needles), HBV vaccination, and regular HBV infection screening. Periodically, if HBV infection must be treated early before cirrhosis, the effectiveness of reducing the risk of liver cancer can be promoted.
4.2 For HCV infected group Treatment with interferon regimen reduces the risk of liver cancer by 70% as well as reduces the risk of liver transplantation by 90%.
5.Once you have liver cancer, can continuing antiretroviral therapy help prevent recurrence?
Direct-acting antiviral therapy is being studied in HCV-infected patients, but interferon regimens that achieve a complete HCV virological response at week 12 increase survival and reduce risk. recurrence after liver tumor resection.
For the group of liver cancer caused by HBV, continuing antiretroviral therapy reduces the recurrence rate by 10% after "radical" treatment measures such as radiofrequency ablation, surgery to remove liver tumors.
6. What progress is there currently in the research/treatment of hepatitis B and C in patients with liver cancer?
Studies are still ongoing to fully understand the effect of good management of hepatitis B and C on the survival time of patients with primary liver cancer. Trials of direct-acting antivirals as well as HCV drugs need further elucidation.Currently, the decision to treat HCV is based on the individual patient, and to initiate treatment if HCV infection has elevated liver enzymes along with the following conditions:

People 18 years of age or older. HCV antibody positive, HCV RNA positive. Compensated liver disease - ie no ascites and/or encephalopathy. Liver, kidney, and marrow functions for hematopoiesis are within permissible limits. Patient wishes to treat the disease (consent to treatment). There are no contraindications to the available therapies. Commonly used regimens in HCV management:
Interferon and Pegylated-Interferon. Interferon plus ribavirin. Direct-acting antivirals: NS3/4 inhibitors (simeprevir, grazoprevir); NS5B inhibitors such as sofosfuvir... are relatively new to the US FDA approved for combination therapy in HCV management.
7. What are the most necessary considerations in managing this patient group?
The biggest note is to pay attention to interactions between drugs used in the management of hepatitis C: between direct acting antiviral drugs as well as drugs used concurrently.
Excerpt from Interview with Dr. Anita Kohli, Director of the Center for Hepatology, Creighton College of Medicine, Phoenix, Arizona, USA. Published in Clinical Advances in Hematology and Oncology, February 2016.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
See more from nobelprize.org; medscape.com