This is an automatically translated article.
Post by Master, Doctor Mai Vien Phuong - Gastrointestinal endoscopy - Department of Medical Examination & Internal Medicine - Vinmec Central Park International General Hospital.G protein-coupled receptors and their signaling pathways are promising targets for the treatment of non-alcoholic fatty liver disease. Accordingly, studies of the gut-hepatic axis are rapidly contributing to the collection of evidence that dysbiosis of the gut microbiota contributes to NAFLD progression through multiple mechanisms.
1. Overview
Nonalcoholic fatty liver disease (NAFLD) is a broad-spectrum disease, characterized by a range of pathologies ranging from simple fatty liver to non-alcoholic steatohepatitis (NASH). NAFLD patients with advanced cirrhosis have a high rate of developing cirrhosis and hepatocellular carcinoma (HCC). NAFLD is often associated with other metabolic disorders, including obesity, diabetes, and insulin resistance. Abnormal accumulation of liver lipids is a major manifestation of NAFLD. Toxic toxicity caused by toxic free fatty acids, such as palmitic acid, cholesterol, and ceramides, contributes to the progression of early NAFLD to advanced NASH and advanced liver disease. However, there is no approved treatment for NAFLD yet.
2. G protein-coupled receptors (GPCRs) play an important role in metabolic disorders and can respond to various extracellular signals, including fatty acids (FAs).
Data increasingly suggest that G protein-coupled receptors and their signaling pathways are promising targets for the treatment of NAFLD. Intestinal-hepatic axis studies are rapidly contributing to the collection of evidence that gut microbiome dysregulation contributes to NAFLD progression through multiple mechanisms, including senescence-associated secretory phenotypes secondary bile acids of hepatic astrocytes (HSCs), product-induced proinflammatory responses via Toll-like receptors, and toxic products.
In addition, bile acids (BA), which are mainly produced in the liver and metabolized by the gut microbiota, have versatile roles in metabolism, including glucose homeostasis, digestion and absorption. lipid absorption in the diet, growth of gut bacteria and liver regeneration. One of the molecular mechanisms for this BA interaction is with the G protein-coupled receptor Takeda 5 and the G protein-coupled bile acid receptor-1 (TGR5/GPBAR1) to regulate lipid and glucose metabolism.
3. G protein-coupled receptors link gut microbiota-mediated connections in a variety of diseases
G protein-coupled receptors have been shown to play essential roles in metabolic diseases, such as NAFLD and obesity, through their function as receptors for bile acids. and free fatty acids. In addition, the G protein-coupled receptor binds the gut microbiota-mediated connections in a variety of diseases, such as intestinal disease, fatty liver, diabetes, and cardiovascular disease.
The latest findings suggest that gut microbiota-derived acetate contributes to hepatic lipidogenesis by converting dietary fructose to hepatic acetyl-CoA and fatty acids. G protein-coupled receptor agonists, including peptides and natural products such as docosahexaenoic acid have been applied to investigate their role in liver diseases. Therapies such as probiotics and G protein-coupled receptor agonists can be applied to modulate G protein-coupled receptor function to ameliorate the hepatic metabolic syndrome.
4. Intestinal microbiota-mediated G protein-coupled receptor expression
The gut-liver axis plays an important role in the development of liver diseases. The gut microbiota-derived metabolites and their associated signaling pathways play an important role in NAFLD development. Rau et al. reported that SCFA-producing bacteria predominated in the fecal bacteria of NAFLD patients, accompanied by high acetate and propionate in fecal metabolites. These metabolites are implicated in immunological features during NAFLD progression. Manipulating the gut microbiome is a promising prevention and treatment strategy for NAFLD.
For example, administration of a bacterial cocktail, consisting of three strains of Bifidobacterium teencentis and three strains of Lactobacillus rhamnosus, alleviated NAFLD symptoms induced by a high-fat, high-cholesterol diet in rats by increasing levels of SCFAs in the gut. . Similar findings have also been achieved in human clinical trials.
5. Intestinal microbiota-derived metabolites contribute to the development of NAFLD
The gut microbiota-derived metabolites that contribute to the development of NAFLD, including SCFAs, endogenous alcohol, and BAs. The intestinal microbiota in the colon is a major source of SCFAs production, which affects lipid and glucose metabolism. It has been shown that both BA and SCFA can activate G protein-coupled receptors to regulate immune responses. Thus, modulating the gut microbiota is an attractive strategy for intervention in liver disease. For example, farnesoid X agonists fexaramine receptors cause lithocholic acid bacteria to produce Acetatifactor and Bacteroides affect hepatobiliary acid synthesis. It is therefore possible to enhance the expression of the intestinal X gene-targeted farnesoid receptor. In hepatocytes, palmitate can be metabolized to sphingosine 1-phosphate (S1P), which binds to different types of S1P receptors (S1PRs) such as S1PR1-3 to activate HSCs into fibroblasts. In addition, S1P may increase the recruitment of bone marrow mesenchymal stem cells by activating S1PRs to produce the proinflammatory cytokines IL-1β, tumor necrosis factor alpha and IL-6, leading to increased accelerate the pathophysiological process of liver disease. The S1P-S1PR1 axis is also implicated in chronic enterocolitis and colitis-associated cancer by regulating IL-6 and the transcription factor STAT3.
6. Products related to gut microbiota also affect other diseases
In addition to liver disease, products related to the gut microbiome influence other diseases, such as inflammatory bowel disease, diabetes, autoimmune disease, and cardiovascular disease, through protein-coupled receptors G. For example, tryptamine, a tryptophan-derived monoamine produced by intestinal bacteria such as Bacteroides thetaiotaomicron, can activate the serotonin 5-HT 4 receptor uniquely expressed in colon epithelial cells to increase ions flow through the colonic epithelium, altering host intestinal transit.
There is increasing evidence that the microbiome plays an important role in influencing host appetite and relative eating behavior. Of note, the gut-hepatic axis is bidirectional because the liver also influences the components of the gut microbiota (Figure 1) by primary bile acids, which can lead to altered appetite
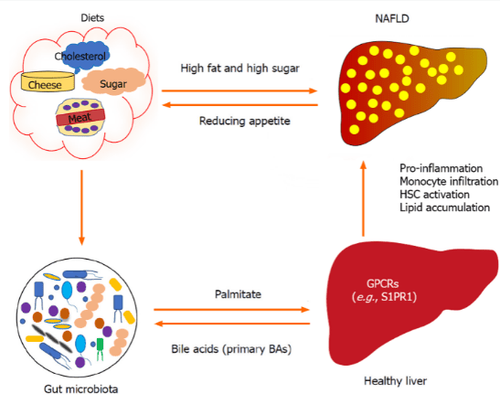
For example, palmitate affects liver function by being converted to sphingosine 1-phosphate in hepatocytes, which can stimulate hepatic astrocyte (HSC) activation and proinflammatory activity via the sphingosine 1 receptor. -phosphate 1 (S1PR1) . In turn, primary bile acids (BA) are synthesized in the liver, which can also affect the composition of the gut microbiota. High-fat and high-sugar diets may induce non-alcoholic fatty liver disease (NAFLD) and alter gut microbiota. Gut microbiota has been shown to impact appetite, and progression of NAFLD may also influence appetite. G protein-coupled receptors: G protein-coupled receptors.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References:Yang M, Zhang CY. G protein-coupled receptors are potential targets for the treatment of nonalcoholic fatty liver disease. World J Gastroenterol 2021; 27 (8): 677-691 [PMID: 33716447 DOI: 10.3748 / wjg.v27.i8.677 ]