This is an automatically translated article.
Posted by Doctor Mai Vien Phuong - Department of Medical Examination & Internal Medicine - Vinmec Central Park International General Hospital.
Abbreviations:
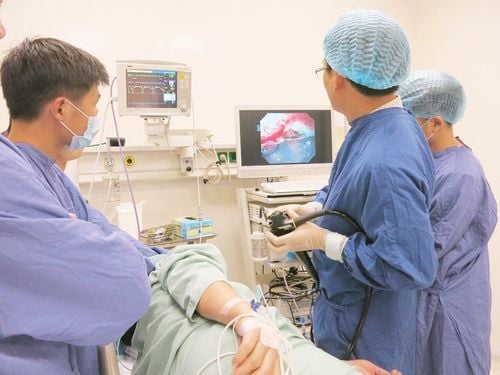
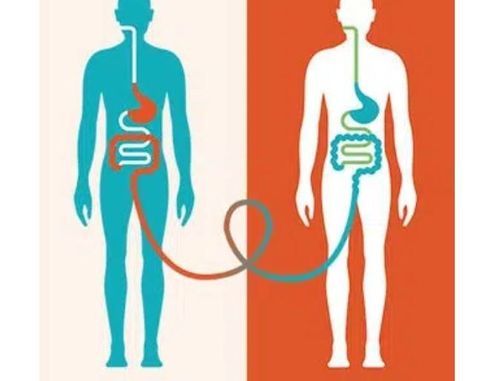
Inflammatory bowel disease (IBD). Ulcerative colitis (UC) Crohn's disease (CD) Fecal transplantation technique (FMT) Fecal transplantation technique (FMT) is the transfer of fecal microorganisms from healthy donors to individuals with certain diseases. certain diseases, through technical methods such as douches, nasogastric or nasojejunal tubes, and oral capsules.
1. What is fecal grafting technique?
FMT was first used to treat pseudomembranous enterocolitis (PMC) in 1958, by Eiseman et al. Subsequent studies have suggested that PMC is caused by infection with the anaerobic bacteria C. difficile, which can cause intestinal dysbiosis. FMT has since been gradually expanded from the preliminary stages of development and testing to be used as an approved treatment for C. difficile infection (CDI) in the clinic, with a high success rate. almost 92%, thus representing an effective treatment compared to broad-spectrum antibiotics. FMT was subsequently used to treat Inflammatory Bowel Disease (IBD) complicated by CDI, and was eventually extended to treat patients with IBD only As a treatment strategy for IBD, FMT was proposed for more than 25 years; however, it has only attracted research interest in the context of IBD in recent years. Several clinical studies have demonstrated promising treatment results for patients in the mildly or moderately active phase of the disease.
2. Efficiency of fecal grafting technique
A systematic review in 2012 found that, of 41 cases of FMT treatment in IBD patients, symptoms were relieved in 76% of patients, 76% of patients were able to discontinue the drug, and 63% of patients. The patient showed signs of remission. However, a later, larger meta-analysis (307 adult patients) did not show a high efficacy rate, reporting that FMT only marginally reduced clinical symptoms in 36% of UC and 50 patients. ,5% of CD patients.
According to a small double-blind randomized trial, conducted to evaluate the safety and efficacy of FMT in UC patients, 41.2% of patients (7/17) achieved the primary endpoint versus 25, 0% control (5/20). Similar results were obtained in a multicenter, double-blind, randomized, placebo-controlled clinical trial, demonstrating efficacy of FMT in only 11 (27%) of 41 UC patients. , with adverse reactions in 32 (78%) cases. ; however, most adverse events were self-limited gastrointestinal complaints. Furthermore, based on 16S rDNA sequence analysis, FMT-induced clinical remission and endoscopic improvement were correlated with the regulation of gut microbiota in active UC.
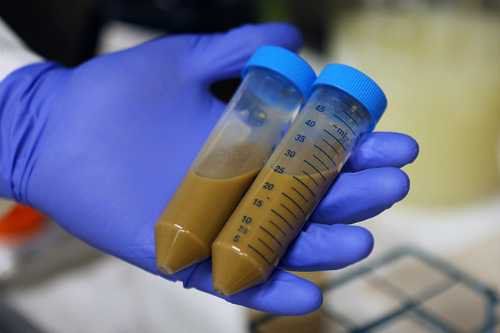
3. The role of stool transplantation in Crohn's disease
Data on FMT-induced reduction of Crohn's disease are less abundant in UC, and randomized controlled trials in CD are incomplete; Only a handful of small, uncontrolled cohort studies have been performed, producing conflicting results. A prospective open-label study found that FMT from healthy donors to active CD recipients reduced symptoms in 58% (11/19) CD patients, all of which showed an increase microbial diversity. In another uncontrolled study in 10 subjects to evaluate FMT, 3 out of 10 CD patients responded to intervention; however, 2 recipients showed serious side effects, and larger controlled trials are needed to confirm the safety and effectiveness of FM.
However, results of a long-term fresh FMT trial performed to evaluate the maintenance effect of symptom relief in CD disease complicated by an inflammatory intra-abdominal mass showed a clinical symptom reduction rate of 48 .0% (12/25), 32.0% (8/25) and 22.7% (5/22), at 6 months, 12 months and 18 months, respectively; Fresh FMT was repeated every 3 months. Furthermore, the long-term clinical effects of varying frequencies of FMT on CD were explored: Treatment intervals of less than 4 months have been shown to effectively maintain clinical benefits derived from FMT firstly. However, the dynamic alterations of gut microbiota and donor-recipient molecular interactions in FMT are still poorly understood. In addition, further studies are needed to determine optimal FMT treatment intensity and match optimal donor-recipient categories based on microbial profiles.
4. Results of clinical trials
Four randomized placebo-controlled trials have been conducted on FMT to date; Of these, three showed significant symptom relief compared with placebo. An open-label study showed that FMT is more applicable to UC than CD patients. However, uniform standards for the route and frequency of FMT use in published trials are not available, which could be a potential reason for the discrepancy between them. Therefore, establishing the optimal FMT route and frequency can provide a solid theoretical foundation and practical guidance for the clinical application of FMT.
Quality control of donor faeces is also important for clinical application and to increase the stability and security of FMT. Recently, the US Food and Drug Administration informed about the potential risk of serious or life-threatening infections when using fecal microbiota for FMT, and stated that Bacterial infections caused by multidrug-resistant organisms. Therefore, the potential danger of FMT prompts researchers to once again focus more on how to increase the stability and security of FMT.
The proposed fecal bank implementation is a promising step towards establishing uniform standards for donor feces. The Dutch donor stool bank was the first stool bank, established in 2015, to provide a standard product for the treatment of recurrent CDI in the Netherlands. Subsequently, FMT experts organized an international consensus conference on fecal banking, which confirmed the feasibility and maneuverability of fecal banking to accelerate FMT adoption in clinical settings. . All in all, it is a solid foundation for the discovery and wide promotion of FMT to ensure recipients receive a safe, reliable, timely and fair distribution of funding.
5. The role of herbs
Although a number of herbal medicines have been used in clinical settings as a complementary and alternative medicine for IBD, their underlying pharmacological modes of action are still little known. The mechanisms by which herbal compounds and prescriptions target the gut microbiota have been described in experimental IBD models. These findings may be just the tip of the iceberg regarding the potential therapeutic mechanisms of herbal therapies.
6. Conclusion
Complementary therapies targeting the gut microbiota are indistinguishable, not following an individual treatment program. However, the gut microbiota composition in different patients is highly individualized. Therefore, it is necessary to screen the microbiome and conduct follow-up investigations of different IBD patients to monitor individual differences in the microbiome and design microbiome-based therapies. individualized organisms to enhance the specificity and selectivity of therapeutic strategies targeting the gut microbiota.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References:
Actis GC, Pellicano R, Fagoonee S, Ribaldone DG. History of Inflammatory Bowel Diseases. J Clin Med . 2019;8:1970. [PubMed] [DOI Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol . 2014;20:91-99. [PubMed] [DOI] Plichta DR, Graham DB, Subramanian S, Xavier RJ. Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell . 2019;178:1041-1056. [PubMed] [DOI Schirmer M, Garner A, Vlamakis H, Xavier RJ. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol . 2019;17:497-511. [PubMed] [DOI] Yue B, Luo X, Yu Z, Mani S, Wang Z, Dou W. Inflammatory Bowel Disease: A Potential Result from the Collusion between Gut Microbiota and Mucosal Immune System. Microorganisms . 2019;7:440. [PubMed] [DOI] Bei Yue, Zhi-Lun Yu, Regulation of the intestinal microbiota: An emerging therapeutic strategy for inflammatory bowel disease, World J Gastroenterol. Aug 14, 2020; 26(30): 4378-4393, [PubMed] [DOI]