This is an automatically translated article.
Posted by Doctor Mai Vien Phuong - Department of Medical Examination & Internal Medicine - Vinmec Central Park International General Hospital.
This article reviews the most recent evidence on interventions that directly or indirectly target the gut microbiome for the treatment of inflammatory bowel disease (IBD) to overcome the current limitations of these therapies. IBD treatment and shed light on personalized treatment options.
Abbreviations:
Inflammatory bowel disease (IBD).
Ulcerative colitis (UC)
Crohn's disease (CD)
Fecal transplantation technique (FMT)
1.Overview
The rapid development of metagenomics (the sequencing and analysis of DNA of microorganisms taken from the environment without culturing them), metabolism and metatranscriptomics (a field of study related to transcripts) genetic code of an organism) provides new insights into the gut microbiota factors that are implicated in inflammatory bowel disease (IBD). Many microorganisms play a role in gut health; they include bacteria, fungi, and viruses that exist in dynamic equilibrium to maintain mucosal homeostasis. Disturbances in the gut microbiota disrupt mucosal homeostasis and are strongly associated with human IBD and murine colitis. Therefore, preventing or correcting microbiome imbalance can be considered as a novel prevention or treatment strategy for IBD.
2.Classical Inflammatory Bowel Disease Treatments
The classical treatments for IBD are diverse, including anti-inflammatory, immunosuppressive and biologic therapies, most of which are adopted and developed in clinical practice. IBD is a persistent, relapsing disease that requires long-term treatment, often resulting in drug-induced side effects. Furthermore, many IBD patients fail to respond to clinically approved drugs, necessitating the development of new therapies or complementary and alternative drugs for IBD. It is worth mentioning that, with the rapid progress in metagenomics, complementary and alternative therapies for IBD based on modulation of the gut microbiota have developed rapidly and preliminary achievements have been reported. Pro/prebiotics, herbal medicinal products, and fecal microbiota transplants (FMTs) are emerging treatment strategies for IBD that target the gut microbiota in a direct or indirect manner, thereby benefiting for gut health.
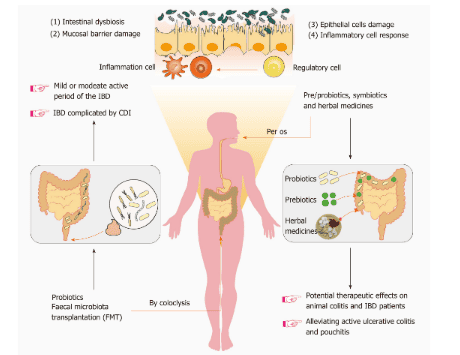
Bacterial-based therapies for inflammatory bowel disease (IBD) can be divided into two categories, namely: Direct microbiome modification [probiotics and fecal microbial cultures (FMT)] and indirect regulation (prebiotics and herbal medicines). Intestinal dysbiosis, mucosal barrier damage, epithelial cell damage, and inflammatory cell responses often coexist in patients with IBD. However, FMT has now been used to trial the treatment of mild or moderate active IBD as well as in patients with IBD complicated by Clostridium difficile infection. Pre/probiotics, symbiotics and herbal medicines show potential therapeutic effects on inflammatory bowel disease in animals as well as in certain IBD patients, particularly with active ulcerative colitis. Therefore, there is a need to screen and design personalized microbiome-based therapies to enhance the specificity and selectivity of a therapeutic strategy targeting the gut microbiota. IBD: Inflammatory bowel disease; FMT: Bacterial stool culture; CDI: Clostridium difficile infection.
3.Probiotics: Live Bacterial Biological Therapy in the Treatment of Inflammatory Bowel Disease
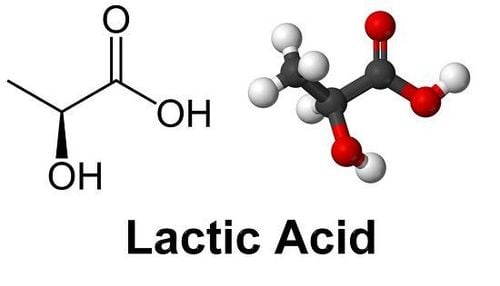
Probiotics were first proposed in 1908 by Nobel laureate Eile Metchnikoff, who also identified the first probiotic agents, lactic acid bacteria, to have the physiological effect of inhibiting “autotoxicity processes.” of the intestine", slows down the aging process of the intestine and produces beneficial effects on human health. Since then, the concept of gut probiotics has been constantly evolving and new strains of beneficial bacteria are still being identified. The latest scientific definition of probiotics, i.e. . “Living microorganisms, when used in adequate amounts, confer a health benefit to the host” was raised in 2014. The probiotic strains discovered to date mostly belong to the group Firmicutes, and include genera Aerococcus, Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Oenococcus, Pediococcus, Streptococcus, Carnobacterium, Tetragenococcus, Vagococcus and Weissella; as well as the genera Bifidobacterium due to Actinobacteria , and Saccharomyces belonging to Eumycota . Lactobacillus, Bifidobacterium and Saccharomyces strains are probiotics with a long history of application and are of great interest to many people. Furthermore, with evolution and innovation in sequencing technology, researchers have discovered new probiotic strains known as “next generation probiotics”, including Akkermansia muciniphila, Propionibacterium spp. And Roseburia spp., with promising applications.
3 consists of 8 strains of live lyophilized bacteria, namely Streptococcus thermophilus, 3 strains of Bifidobacteria (B. longum, B. breve, and B. infantis) and 4 strains of Lactobacilli ( L. paracasei , L. plantarum , L. acidophilus and L. delbrueckii subspecies bulgaricus ). VSL
3 has long been used in clinical settings for the treatment and remission of IBD. One study confirmed that VSL
3 achieved remission in patients with mild to moderate active UC, with high safety and efficacy. Furthermore, in a recent systematic meta-analysis, VSL
3 also demonstrated efficacy in reducing UC active and pouch inflammation, and may effectively protect against its recurrence in the early phase. static disease phase; however, its potential use in CD patients has not been demonstrated. The exact effects of probiotics in the gut are still unclear.
4.The potential impact of probiotics on the gut
The elucidation of the mechanisms by which probiotic bacteria exert a protective effect in IBD is important for determining optimal treatment strategies. The potential effects of probiotics on the gut can be classified into 4 categories:
(1) Probiotics modulate immune responses and inhibit inflammatory responses by mediating several signaling pathways signaling, such as the Toll receptor-like signaling pathway;
(2) Probiotics inhibit or directly eliminate intestinal pathogenic microorganisms by competing for nutrients and intestinal epithelial adhesion sites, and secreting antibacterial substances;
(3) Probiotics help maintain intestinal epithelial homeostasis by promoting tight junction (TJ) formation, promoting mucus production, and resisting epithelial cell death;
(4) Probiotics can directly affect the metabolism of the intestinal microbiota and the host, thereby promoting the regulation of colon cell proliferation and elimination of toxic substances. from the intestines.
5.Herbal Compounds and Prescriptions
There are some safety concerns with long-term use of common drugs (eg, anti-inflammatory, immunosuppressive and biologic therapies), which has increased interest in Traditional medicine to treat IBD. As a result, more and more researchers are turning their attention to traditional medicine to identify potentially curative compounds in Chinese herbal medicine and/or traditional prescriptions. To date, various potent compounds have been found, some of which have been shown to reduce intestinal inflammation, at least in part by modulating the gut microbiota. However, the meager data may actually reflect the treatment's effectiveness in human clinical trials.
Wide variety of natural compounds derived from herbs, including herbal polysaccharides, polyphenols, flavonoids, saponins and alkaloids. Furthermore, herbal polysaccharides and polyphenols, which are present in various Chinese herbs and are almost exclusively absorbed in the colon, have not yet been included in the prebiotic category. Herbs containing polysaccharides include several Chinese medicines such as American ginseng and wild wolfberry, both of which have been shown to modulate intestinal dysbiosis and minimize intestinal inflammation in rats. Polyphenols in herbal medicine include anthocyanins, catechinic acid, ellagic acid and gallic acid, which can be converted to bioactive metabolites by intestinal microorganisms. Therefore, the modification of the microbial community structure is beneficial for the gut.
6.The role of herbs in changing the intestinal microbiota
Other prebiotic-free natural ingredients have also been shown to reduce intestinal inflammation in rats with colitis by selectively altering the gut microbiota; however, it has not been demonstrated whether these compounds participate in bacterial metabolism.
Certain natural alkaloids, such as berberine, palmatine and evodiamine, have been shown to ameliorate experimental colitis in an IBD model by improving the relative abundance of the gut microbiota , as well as increasing the abundance of Bacteroidetes and Firmicutes and decreasing the abundance of Proteobacteria, thereby maintaining the homeostasis of the gut microbiota. A natural limonoid compound, obacunone (100 mg/kg/day orally in rats) abundantly distributed in Chinese Phellodendron and Tetradium ruticarpum, exhibits a modulating effect on the dysregulated gut microbiota of IBD rats .
Others, such as Indigo naturalis (200 mg/kg/day orally in rats) and salvianolic acid (8 mg/kg/day by tail vein injection in rats), also target the microflora. gut flora, which have beneficial effects on gut health. Furthermore, recent studies have also demonstrated the effectiveness of some traditional Chinese prescriptions: As a traditional compound, Bawei Xileisan (200 or 400 mg/kg/day by mouth) consists of 8 Chinese medicines, including watermelon, calcite, rubber stone, pear medicine, fenugreek, Dryobalanops aromatica Gaertn. f., ammonium chloride, and Indigo naturalis, and has been shown to reduce colitis in a UC rat model primarily by restoring the Th17/Treg imbalance and improving the abundance of Lactobacillus. Peony rhubarb decoction is another Chinese prescription that increases Butyricicoccus pullicaecorum abundance and SCFA, thereby alleviating pathological changes in rats with colitis. Recently, Pyungwi-san (669.1 mg/kg/day orally) was found to protect against DSS and C. difficile-associated colitis, and this mechanism is involved in its restoration. balance in the gut microbiota.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.