This is an automatically translated article.
The article was written by Pharmacist Nguyen Thi Bich Phuong - Clinical Pharmacist, Faculty of Pharmacy - Vinmec Ha Long International General HospitalFlixotide is a medicine containing fluticasone propionate, a corticosteroid used in the treatment and prevention of asthma and chronic obstructive pulmonary disease.
1. Uses of Flixotide
The drug may relieve symptoms and reduce the frequency of asthma exacerbations and chronic obstructive pulmonary disease (COPD) exacerbations in patients previously treated with bronchodilators alone or with prophylactic therapy. another room.
Currently, the Drug Administration of Vietnam has licensed the circulation of several dosage forms of Flixotide: Flixotide Nebules 0.5mg/2ml aerosol inhalation suspension and metered dose nebulizer (metered dose inhaler) Flixotide Evohaler 125mcg contains 120 spray doses...
2. Dosage and usage of Flixotide
For Flixotide Evohaler metered dose inhaler Adults and children over 16 years: 100 - 1000 micrograms × 2 times/day. Patients should use an appropriate starting dose depending on the severity of the disease:
+ Mild asthma: 100-250 micrograms × 2 times/day.
+ Moderate asthma, chronic obstructive pulmonary disease: 250-500 micrograms × 2 times/day.
+ Severe asthma: 500-1000 micrograms × 2 times/day.
The dose can then be adjusted until control is achieved or reduced to the lowest effective dose, depending on individual patient response.
Children 4 years and older: 50-200 micrograms × 2 times/day. Many children with asthma are well controlled when using doses of 50-100 micrograms × 2 times/day. For children in whom this dose is not sufficient to control asthma, a therapeutic effect can be achieved by increasing the dose to 200 micrograms twice daily. The appropriate starting dose should be given to the child depending on the severity of the disease. The dose can then be adjusted when control is achieved or reduced to the lowest effective dose depending on individual patient response.
Children 1 to 4 years: 50-100 micrograms × 2 times/day administered through a pediatric spacer with a mask (e.g. BABYHALERTM) achieving optimal control of asthma symptoms. How to use:
Check the product: + Remove the cover, check the inside + outside to make sure the mouthpiece is clean and free of foreign objects.
+ For the first use / after a period of non-use (≥ 7 days), it should be tested by spraying 2 puffs into the air.
Maneuver: (Position of taking medicine: standing or sitting upright)
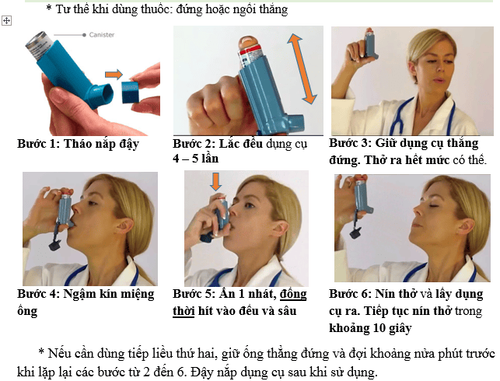
Step 1: Remove the cap
Step 2: Shake the instrument 4-5 times
Step 3: Hold the tool upright, exhale as much as you can
Step 4: Close the mouth of the tube
Step 5: Press 1 time , at the same time inhale evenly and deeply
Step 6: Hold your breath and remove the tool. Continue holding your breath for about 10 seconds
If a second dose is needed, hold the tube upright and hold for about half a minute before repeating steps 2-6. Close the tool cap after use.
Note: Patients need to rinse their mouth with water and spit it out after use.
Cleaning of instruments: Recommended ≥ 1 time/week: remove the ampoule from the instrument, open the cap of the instrument, wash it with warm water, then dry from the inside out (it is recommended to let it dry naturally). Put the tube back in and close the cap. For children: Use with spacer and mask (suitable for children under 12 years old). The steps to use are almost the same as above, but it is important to shake the bottle before inserting it into the spacer, clean the mouth area covered by the mask after use.
For Flixotide Nebules aerosol inhalation suspension 0.5mg/2ml Asthma:
Adults and adolescents over 16 years old: 500 - 2000 micrograms x 2 times/day. Children and adolescents aged 4 to 16 years: 1000 micrograms x 2 times/day. Patients should be given nebulized Flixotide at an initial dose appropriate to the severity of the disease. The dose should then be adjusted until the disease is controlled or reduced to the lowest effective dose depending on individual patient response. For the treatment of acute exacerbations of bronchial asthma, the maximum dose should be used for up to 7 days after the exacerbation. Then consideration should be given to reducing the dose.
How to use: Flixotide Nebules should be used in the form of an aerosol created by a gas nebulizer according to the instructions of the doctor.
Aerosol medications are inhaled by mouth and must use a mouthpiece. If necessary, use a mask that can be inhaled through the nose.
To facilitate the use of a small amount of suspension or, if it is necessary to prolong the delivery time, the suspension for nebulization can be diluted with sodium chloride injection for injection immediately prior to use.
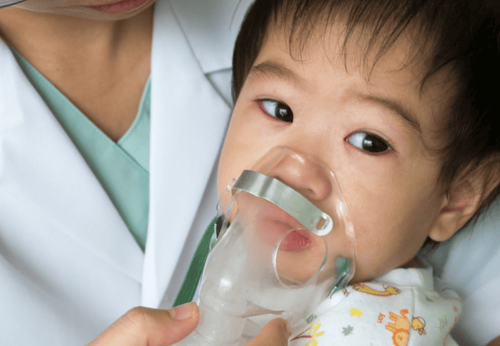
Có thể sử dụng mặt nạ khí dung và pha thuốc theo đúng chỉ định của bác sĩ
3. Flixotide side effects Serious but uncommon reactions: Allergic reaction rash, itchy rash, redness, blistering, peeling, difficulty breathing, swelling of mouth, lips, tongue, throat; symptoms of infection such as: fever, chills, earache, sore throat, cough, lots of phlegm. The medicine can cause very severe breathing difficulties soon after taking it. Sometimes, this can be life-threatening. If you experience worsening shortness of breath or wheezing, use reliever medication and call your healthcare provider right away.
Common: Oral candidiasis, throat, hoarseness, bruising. Some other undesirable effects: Cushing's syndrome, Cushing-like manifestations, adrenal suppression, growth retardation in children, decreased bone mineral density, cataracts, glaucoma; Increased blood sugar; Anxiety, sleep disturbances and behavioral changes, including hyperactivity and irritability (mainly in children).
4. Things to keep in mind when using Flixotide Control of asthma and chronic obstructive pulmonary disease should follow a ladder program and patient response should be monitored clinically and through functional tests lung. Increased use of beta agonists; Short-acting inhalers for asthma symptom control indicate poor asthma control. In these cases, the patient's treatment regimen should be re-evaluated and the appropriate drug selected. Sudden and worsening deterioration in asthma control is potentially life-threatening for the patient, so an increase in corticosteroid dose should be considered. In patients considered at risk, daily peak flow monitoring is recommended. The metered-dose inhaler should not be used during an acute asthma attack, but only for routine long-term control. Patients will need to take a short-acting inhaled bronchodilator to relieve acute asthma symptoms. The child's height should be regularly checked when long-term inhaled corticosteroids are used in children. To minimize unwanted effects such as: Oral candidiasis, hoarseness, it is necessary to rinse the mouth and spit it out after taking the medicine. Patients should not arbitrarily increase, decrease the dose or stop the drug suddenly. If you miss a dose, skip the dose and take it at your daily schedule, do not add a dose on your own or take a double dose to make up for the missed dose.
Người bệnh chỉ nên dùng thuốc Flixotide khi có chỉ định của bác sĩ điều trị
Need to store the medicine in a dry place, not in the bathroom or high humidity area. Do not use metered dose inhaler, nebulizer suspension tube for oral administration or injection. Because this is a special dosage form, when the patient uses the drug exactly as the doctor recommends, but the treatment effect is poor, it is necessary to re-check the patient's drug manipulation and technique. If you are using more than one spray/inhaler, ask your doctor or pharmacist about the effects, usage, and timing of each type.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References
Product information leaflet Vinmec Uptodate drug user manual, accessed July 29, 2020