This is an automatically translated article.
With highly selective competitive neuromuscular blocking effect, Atracurium muscle relaxant is used by doctors as an adjunct to general anesthesia to perform intubation and relax skeletal muscle in surgery or mechanical ventilation. The following article will clarify information about the drug Atracurium and related issues.
1. The effect of the drug Atracurium
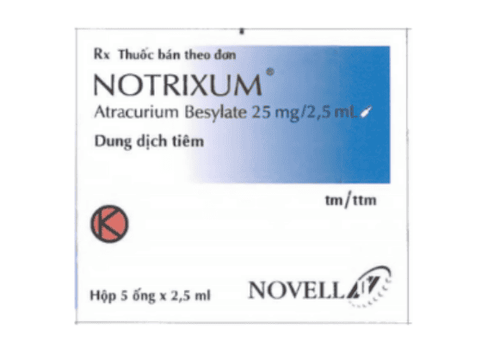
1.1. What is Atracurium? Atracurium, trade name Tracrium®, is a muscle relaxant indicated in competitive and highly selective non-depolarizing neuromuscular blocking. It is used as an adjunct to anesthesia to enable tracheal intubation and skeletal muscle relaxation during surgery or controlled ventilation in many medical procedures. Atracurium helps to support mechanical ventilation of patients in the intensive care unit (ICU), and induces skeletal muscle relaxation during surgery and to facilitate mechanical respiratory control after anesthesia.
In addition, Atracurium is suitable for maintaining muscle relaxation during cesarean section because it does not cross the placenta at clinically significant concentrations following administration at the recommended doses.
1.2. What are the side effects of Atracurium? Atracurium is used as an adjunct to general anesthesia to facilitate endotracheal intubation and assist ventilation. Atracurium besylate has no direct effect on intraocular pressure and is therefore suitable for use in ophthalmic surgery.
2. Usage of the drug Atracurium
2.1. Dosage of Atracurium Adults: The dose of Atracurium besylate must be carefully adjusted according to individual response. In order to accurately monitor muscle relaxation and minimize the possibility of overdose, a peripheral nerve stimulator should be used to monitor nerve block and recovery in anesthetized patients.
Initial dose:
Intubation is 0.4 - 0.5 mg/kg. After this initial dose, endotracheal intubation for non-emergency surgery can be performed within 2 to 2.5 minutes in most patients and maximal neuromuscular blockade usually occurs within 2 to 2.5 minutes. 3-5 minutes. When used concurrently with general anesthesia, this initial dose usually provides sufficient clinical neuromuscular block for 20 to 35 minutes. Recovery is up to 25% of baseline after about 35-45 minutes and to 95% about 1 hour after administration. The manufacturer recommends that the initial adult dose be reduced by approximately 33% (i.e., 0.25 - 0.35 mg/kg) when the drug is given after stable anesthesia with enflurane or isoflurane, a reduction of less than approx. 20% under halothane anesthesia, since halothan has only minimal effect on the neuromuscular blocking activity of atracurium besylate.
Maintenance dose - Interval IV:
To maintain neuromuscular blockade during prolonged surgery, the dose is individually adjusted and the usual dose in adults is 0.08 - 0 ,1 mg/kg. In patients receiving general anesthesia, the first maintenance dose is usually needed 20 to 45 minutes after the initial dose. Repeated maintenance doses may be given at relatively regular intervals of 15 to 25 minutes in patients receiving general anesthesia. When enfluran or isofluran is used for anesthesia or when a higher maintenance dose of atracurium besylate is used, this maintenance dose can be administered at longer intervals. Continuous Intravenous Infusion:
In protracted surgeries, a continuous intravenous infusion of atracurium besylate may be possible after rapid intravenous administration of the initial dose. Begin the infusion only after recovery from the initial intravenous dose has begun. Continuous intravenous infusion at an initial rate of 9 - 10 micrograms/kg/min, and then maintained at an infusion rate of 5 - 9 micrograms/kg/min for patients under general anesthesia. The infusion rate is reduced by approximately 33% under steady-state anesthesia with enflurane or isofluran. Children: The recommendations for the starting dose, maintenance dose, and continuous intravenous infusion rate of atracurium besylate for children 2 years of age and older are the same as for adults.
Neonates, breastfed infants, children < 2 years old:
Initial dose:
0.3 - 0.4 mg/kg followed by a maintenance dose of 0.3 - 0.4 mg/kg if needed to maintain the rhythm neuromuscular block. Or: Continuous infusion: 0.6 - 1.2 mg/kg/hour or 10-20 micrograms/kg/min. Other subjects: For people with renal failure: No dose adjustment is required.
The dose may need to be increased in patients with burns.
2.2. How to take Atracurium Atracurium besylate is given by rapid intravenous injection or intravenous infusion. In protracted surgeries, a continuous intravenous infusion may be used. For continuous intravenous infusion, Atracurium besylate injection must be diluted to a concentration of 0.2 or 0.5 mg/ml in 5% dextrose injection solution; dextrose 5% and sodium chloride 0.9%, or sodium chloride 0.9%.
To avoid worrying for the patient, only give medicine when the patient loses consciousness.
3. Contraindications of the drug Atracurium
Do not administer Atracurium besylate to patients with known hypersensitivity to the drug or to any component of the preparation.
4. Notes when using Atracurium
4.1. General Precautions Atracurium besylate can cause respiratory depression and respiratory paralysis. Atracurium besylate should only be used by clinicians experienced in the use of neuromuscular blocking agents; staff and facilities for intubation for controlled respiration must be available. A cholinesterase inhibitor such as neostigmine, pyridostigmine, or edrophonium must be available to treat respiratory failure caused by atracurium besylate.
Patients receiving prolonged Atracurium besylate should be monitored continuously in the emergency department. Do not take additional doses before a definite response to nerve stimulation testing has been obtained. If there is no response to nerve stimulation, the drug should be withheld until response returns. Atracurium besylate is less likely to stimulate histamine release than some other muscle relaxants, but should still be used with caution and at a lower initial dose in people at high risk for histamine release (cardiovascular disease or pre-existing conditions). history of anaphylactic or asthmatic reactions). Be alert for the development of malignant hyperthermia and be ready to handle in the anesthetized patient. Resistance to atracurium may develop in burn patients, so the dose should be increased in this patient.
Because atracurium besylate has very little cardiovascular effect, especially at recommended doses, it is not effective against bradycardia caused by many anesthetics or by parasympathetic stimulation. Bradycardia during anesthesia may be more common than with other neuromuscular blocking agents.
Atracurium besylate should be used with extreme caution in patients with severe myasthenia gravis, as this disease may increase the neuromuscular blocking effect of the drug. The degree of neuromuscular blockade should be monitored with a peripheral nerve stimulator. Also monitor the degree of such neuromuscular blockade in patients with severe electrolyte disturbances or systemic metastatic cancer.
4.2. Precautions for pregnant women There are no adequate and well-controlled studies of the use of Atracurium besylate in pregnant women to date, so it should be used during pregnancy only when the benefits are weighed against the possible risk to the fetus.
As it is not known whether Atracurium besylate is distributed in breast milk, it must be used with caution in nursing women
5. Atracurium side effects
Although it may be rare, some people can experience very severe and sometimes fatal side effects when taking the medicine. Tell your doctor or get medical help right away if you have any of the following signs or symptoms:
Signs of an allergic reaction, such as a rash; hives; itchy; red, swollen, blistering, or peeling skin with or without fever; wheeze; tightness in the chest or throat... Fast or slow heart rate.
6. How to store Atracurium
Store in the refrigerator between 2°C-8°C. Do not freeze
Store in original packaging to protect from light.
Storage conditions of diluent
Unopened: 24 months from date of manufacture
Opened: must be used immediately after opening.
Knowing the information about Atracurium and its use will help patients know how to use the drug safely and effectively for the treatment process. If you have any further questions, you should talk to your doctor before taking them.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.