This is an automatically translated article.
Article written by Dr. Pham Anh Tuan, Vinmec Stem Cell Research Institute and Gene Technology
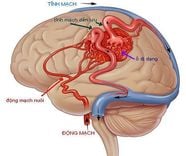
Our DNA determines a large part of the risk of Alzheimer's disease, but it is not clear how many genetic risk factors contribute to the disease. A research team led by Professor Bart De Strooper (VIB-KU Leuven) and Dr Mark Fiers has now shown that many risk factors affect the brain's preserving cells - microglia- and more specifically is their response to amyloid-beta, one of the proteins that accumulate in the brains of Alzheimer's patients. The individual effects of small genetic variations may be negligible, but the combination of hundreds of such subtle changes can upset the balance and cause disease.
Why do some people get Alzheimer's while others don't, even in very old age? Although, through decades of research, we still do not know the complete answer to this question. Epidemiological studies show that about two-thirds of a person's risk of developing Alzheimer's disease is genetically determined. Several dozen risk genes have been identified; however, recent evidence suggests that there may be hundreds of additional genetic variations, each of which contributes a small but significant part to disease risk. .
1. From risk genes to disease expression mechanisms
Bart De Strooper (VIB-KU Leuven) has been studying the mechanism of Alzheimer's disease for decades. His team is trying to understand what genetic risk is involved to help us understand how the disease develops in the brain. Two important questions arise from countless genetic studies: First, what is the link between Alzheimer's risk genes and the amyloid-beta plaques or tau plexuses that we find in the brains of people with Alzheimer's disease? ; and second, or are they all involved in a central cellular/molecular signaling pathway, or are they present in parallel pathways, and both lead to Alzheimer's disease?
DNA của chúng ta quyết định một phần lớn nguy cơ mắc bệnh Alzheimer
The researchers investigated when these genes were expressed and specifically whether they responded to tau or amyloid-Beta pathology. “When it comes to risk, you always need to take context into account,” explains Mark Fiers, co-author of the study. "If you don't wear a seat belt in the car, it doesn't matter as long as you don't have an accident." With this in mind, the researchers aimed to understand under what circumstances the genetic risk for Alzheimer's disease emerges. Most people develop some degree of Alzheimer's disease in the brain, ie having amyloid-beta plaques and tau plexuses, however, some people remain cognitively healthy despite loads, Fiers said. disease severity is high, while others develop Alzheimer's symptoms fairly quickly.
Annerieke Sierksma, postdoctoral researcher in De Strooper's lab said: "For more insights, they examined gene expression in two different mouse models of Alzheimer's disease, one in amyloid-beta expression and tau pathology pattern, at different ages. Research has determined that many genes associated with Alzheimer's risk are specifically responsive to amyloid-beta but not to tau pathology.
2. Activation state in microglia
The team identified 11 novel risk genes that were significantly up-regulated in the face of increased amyloid-beta levels. All of these genes are expressed in microglia, cells that play an important role in protecting the brain.
11 gen ảnh hưởng đến nguy cơ mắc bệnh Alzheimer đều được biểu hiện trong microglia
Study confirms that microglia exposed to amyloid-beta strongly switch to an activated state, which occurs to a much lesser extent in tau mice. These new results indicate that a large part of the genetic risk of Alzheimer's disease is related to the response of microglia to amyloid-beta.
3. Genetic risk factors
Should we reconsider the classical gene-based view, where certain mutations or genetic variations lead to disease? De Strooper argues: "A single genetic variation in a functional network will not lead to disease. However, multiple variants in the same network can cause a disturbance in the balance leading to disease. Such a hypothesis could also explain the conundrum that some people have a lot of amyloid-beta in their brain but do not develop clinical symptoms."
Nhiều biến thể trong cùng một mạng lưới mới có thể là nguyên nhân gây ra bệnh
Fiers further points out that, although amyloid-beta may be the causative agent of the disease, the genetic impact of microglia and possibly other cell types determines whether a pathological condition occurs. . Therefore, determining which genetic variations are important for such network disorders and how they alter gene expression remains the next major challenge.
4. Test on mice?
De Strooper explains that postmortem brain tissue data collection provides only specific insights into disease progression stages and does not allow delineation of cause-effect relationships. On the other hand, while the transgenic mouse models only partially summarize the disease, they do allow for a detailed understanding of the early stages of the disease, which are closely related to the measures taken. preventive treatment.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.