This is an automatically translated article.
The active ingredient Taver is Carbamazepine. It is indicated for the control of seizures and paroxysmal trigeminal neuralgia. The following article provides you with information about the uses and notes when using Taver.
1. What is Taver?
Taver drug has the main active ingredient is Carbamazepine, the drug is made in the form of tablets with a strength of 200mg.
Carbamazepine's mechanism of action in Taver is chemically related to tricyclic antidepressants, but is still not fully known. The anticonvulsant effect of Carbamazepine is related to a decrease in neuronal excitability and synaptic blockade, mainly by restricting neurons to maintain sequential, high-frequency, action potential initiation, in addition to carbamazepine effects. presynaptic to block neurotransmitter release, reducing synaptic conduction.
Carbamazepine is effective against paroxysmal pain in patients with trigeminal neuralgia, epilepsy, and alcohol withdrawal. Carbamazepine reduces the risk of spasticity, increases the seizure threshold, and reduces alcohol withdrawal symptoms.
2. Indications and contraindications to the use of Taver
2.1. Indications Taver is indicated in the following cases:
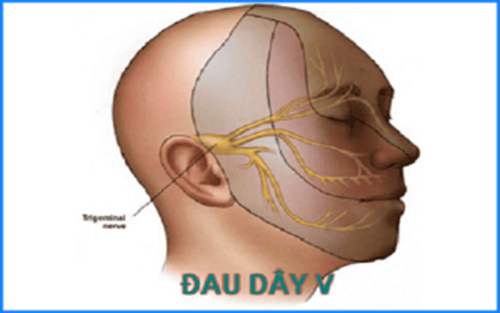
Control seizures, partial seizures, generalized stiffness. Paroxysmal trigeminal neuralgia. Manic-depressive psychosis in patients unresponsive to lithium therapy. 2.2. Contraindications Brivaracetam is contraindicated for use in the following cases:
Hypersensitivity to Carbamazepine, or to any ingredient in the formulation of the tablet or structurally related drugs such as tricyclic antidepressants . Patients with active liver disease or severe liver failure, history of acute porphyria or history of bone marrow failure, atrioventricular block. Do not take Taver concurrently with an MAO inhibitor. Before taking Taver, MAO inhibitors should be discontinued for at least 14 days, and preferably longer if the patient's clinical condition does not permit.
3. How to take Taver
3.1. Dosage Epilepsy
Adults: Initial dose is 100-200mg/time x 1-2 times/day. Dosage may be titrated in increments until the desired clinical response is achieved, usually in the range of 800 to 1200 mg. Some patients can take up to 1600 - 2000mg. Elderly: There is no evidence to differ in the pharmacokinetics of Carbamazepine in the elderly, and therefore no dose adjustment is necessary. However, because of the potential for possible drug interactions, caution should be exercised when dosing Carbamazepine in the elderly. Children: divide the following doses into equal doses. 1-5 years old: 200-400mg/day. 5-10 years old: 400 - 600mg/day. 10-15 years old: 600 - 1000mg/day. Clinically valuable epilepsy drugs should be used as monotherapy; however, for combination therapy of Taver with other drugs, a gradual dose escalation regimen should be used.
Where Taver is indicated as an adjunct to pre-existing antiepileptic therapy, a gradual dose escalation regimen should be instituted or, if necessary, the dose of the other antiepileptic drug may be adjusted.
Trigeminal neuralgia
Initial dose 200-400mg/day (100mg x 2 times/day in the elderly), adjust dose gradually until pain symptoms disappear. For most patients, effective pain relief is achieved at a dose of 200mg x 3-4 times/day, some patients may have a total daily dose up to 1600mg. The dose of Taver should then be gradually reduced to the lowest maintenance dose that can control symptoms.
Prevention of manic-depressive disorder in patients unresponsive to lithium therapy
Initial dose of Taver 200mg twice daily. The dose of Taver is then gradually increased until symptoms are controlled, some patients may have a total daily dose of up to 1600 mg. For most patients, doses between 400 and 600 mg/day have been found to be appropriate.
3.2. Overdose and missed dose Taver The first signs of overdose usually appear about 1-3 hours after taking the drug. The most prominent are the symptoms of neuromuscular disorders. Milder cardiovascular disorders and serious cardiac events occur only when Taver is used in very high doses > 60g. If the patient is taking concomitant tricyclic antidepressants, barbiturates or hydantoin, or drinking alcohol, the signs of acute carbamazepine toxicity may worsen or change.
The prognosis of severe poisoning depends mainly on the rapid elimination of Taver, which can be by gastric lavage, induction of vomiting or reduction of carbamazepine absorption by appropriate measures (100g of activated charcoal, then every 4 hours take 50g until recovered).
If the above measures cannot be applied, the patient should be transferred immediately to the hospital to ensure the patient's vital functions. There is currently no specific antidote for Carbamazepine. In addition, apply symptomatic and supportive measures such as monitoring pupillary reflex, temperature, respiratory function, heart (electrocardiogram monitoring), blood pressure, kidney function.
If you forget to take a dose of Taver, take it as soon as possible. If it is almost time for the next dose, skip the missed dose and take the next dose as scheduled. Also, do not take a double dose of Taver.
4. Undesirable effects when using Taver
Undesirable effects when using Taver with very common or common frequency such as somnolence, fatigue, dizziness, ataxia, double vision, headache, nausea or vomiting and other reactions skin allergies.
These undesirable effects usually occur when starting carbamazepine treatment or when used in the elderly, or when starting with high doses. The adverse effects associated with the dose of Carbamazepine usually subside after a few days, possibly after dose reduction or spontaneously.
The occurrence of side effects on the central nervous system indicates an overdose of the drug or due to fluctuations in the plasma concentration of Carbamazepine. In this case, it is necessary to monitor the plasma concentration and divide the daily dose of Carbamazepine into several smaller doses.
5. Notes when using Taver
Before using Taver, it is necessary to perform blood tests including complete blood count, serum iron; and should be evaluated periodically thereafter. Taver should be discontinued if there is evidence that the patient develops neutropenia, signs of significant bone marrow depression, or is accompanied by clinical signs such as fever or sore throat. Patients are advised to notify their physician if any of the following symptoms occur: fever, itching, sore throat, mouth ulcers, purpura or petechiae, easy bruising.
In addition, patients should check baseline values of liver function before using Taver and periodically throughout treatment. Discontinue use of Taver if the patient develops signs of a severe skin reaction such as Stevens-Johnson syndrome or toxic epidermal syndrome (Lyell syndrome).
Caution should be exercised when using Taver in patients with mixed seizures such as atypical and atypical absence seizures. Since Carbamazepine may cause acute seizures in these patients, if an acute seizure occurs, the drug should be discontinued. Abrupt discontinuation of Taver may cause the patient to enter a state of epileptic seizures. In cases where immediate discontinuation of the drug is necessary to switch to another antiepileptic therapy, additional therapy such as intravenous phenytoin or diazepam should be used.
Pregnancy: There are no adequate and well-controlled studies of the use of Carbamazepine in pregnant women. Carbamazepine can cross the placental barrier and accumulate in fetal tissue. Carbamazepine should therefore be used with caution in pregnant women with epilepsy. Whenever clinically permissible, carbamazepine monotherapy should be indicated in women of childbearing potential, because of the higher incidence of birth defects in the infants of women receiving combination therapy than in children of women. women taking monotherapy.
Lactation: Carbamazepine is excreted in breast milk. The benefits of breastfeeding and the possible side effects for the infant should be weighed against the use of Taver in the mother.
6. Drug interactions
Drug interactions with carbamazepine are almost entirely related to the enzyme-inducing properties of the drug. Co-administration of Carbamazepine with Primidone, Ethosuximide and Clonazepam increases the metabolism of liver enzymes that may decrease steady-state blood concentrations of carbamazepine and increase the rate of metabolism of these drugs. Propoxyphene, Troleandomycin and valproic acid decrease the clearance of Carbamazepine and increase steady state blood concentrations. Lithium: Concomitant use with Carbamazepine may increase the risk of neurotoxic side effects, even if the blood levels of Carbamazepine and Lithium are below toxic levels. Concomitant use of MAO inhibitors with carbamazepine has resulted in hypertensive crisis, high fever, severe convulsions, and death. Discontinue MAO inhibitors at least 14 days before starting carbamazepine or vice versa.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.