This is an automatically translated article.
What does Fludarabine do, is it a cancer treatment? In fact, Fludarabine is an anti-metabolite antineoplastic agent used to treat chronic lymphocytic leukemia.
1. What are the effects of Fludarabine?
Fludarabine belongs to the group of anti-metabolic anticancer drugs, with the main ingredient being fludarabine phosphate. This active substance has an inhibitory effect on DNA synthesis.
Fludarabine is prepared in the form of lyophilized powder in a 50mg vial to mix with distilled water for injection into a solution of 25mg/ml, or ampoules/vials for 2ml of 25mg/ml solution. Fludarabine is indicated for the treatment of chronic lymphocytic leukemia in cases of unresponsiveness or progression of disease even while taking alkylated drugs.
2. Usage and dosage of Fludarabine
Fludarabine is administered by intravenous infusion. The treatment, drug infusion will be done by medical staff or doctors. While taking the drug, the patient should follow the doctor's instructions.
Dosage of Fludarabine in adults is as follows:
Treatment of chronic lymphocytic leukemia: The recommended starting dose is 25 mg/m2 body surface area, administered as a once-daily infusion and continued for a long period of time. 5 days. Alternatively, a dose of 30mg/m2 of body surface area can be used, administered as an infusion once a day and also continuously for 5 days. In patients susceptible to toxicity, dosage adjustment should be considered. Each batch is 28 days apart. Treatment of high-risk acute myeloid leukemia: Fludarabine 30 mg/m2 body surface area/day intravenously, administered as a loading dose for 5 days and then maintained for 4 days at the same dose. Treatment of acute myeloid leukemia, drug-resistant: Intravenous 30mg/m2 body surface area/day, used in combination with cytarabine and filgrastim for 5 days. Treatment of non-Hodgkin lymphoma: Combination of fludarabine and other drugs at a dose of 25mg/m2 body surface area/day, used for 3 days and 21 days cycle. Treatment of Waldenstrom's disease: Intravenous 25mg/m2 body surface area/day, used for 5 days and 28 days cycle. Anti-rejection therapy in stem cell transplantation: Intravenous fludarabine 30mg/m2 body surface area/dose, 6 doses for 10 days prior to stem cell transplantation. Alternatively, 30 mg/m2 body surface area/dose may be administered intravenously, in combination with busulfan for 5 days and 6 days before the start of transplantation. Dosage of Fludarabine in children is as follows:
Treatment of acute myeloid leukemia: Intravenously 10.5mg/m2 body surface area, inject the whole dose over 15 minutes, within 48 hours following the dose. 30.5mg/m2 body surface area/day. Treatment of acute myeloid or lymphocytic leukemia: The specific adult dose of Fludarabine is as follows: Fludarabine 10.5 mg/m2 body surface area, administered as a whole dose over 15 minutes, over the next 48 hours. at a dose of 30 mg/m2 body surface area/day. Anti-rejection treatment: 30mg/m2 body surface area/dose, administered in 6 doses for 7-10 days prior to transplantation. Fludarabine dosage in patients with renal impairment is recommended to be reduced by 20% if renal impairment is moderate and requires close monitoring. Patients with severe renal impairment are advised not to take the drug.
Overdosage of Fludarabine can occur at doses of 96mg/m2 or more (for 5-7 days) with neurotoxic manifestations and lead to death. Although the maximum allowable dose is up to 40 mg/m2 (for 5 days), due to limited data, care should be taken not to exceed the recommended dose.
There is currently no specific antidote for Fludarabine overdose. To control, the patient is asked to stop taking the drug and apply symptomatic and supportive measures. In some necessary cases such as bone marrow failure, blood transfusion and hematology can be performed.
3. Fludarabine side effects
Fludarabine can cause some unwanted side effects with the following frequency:
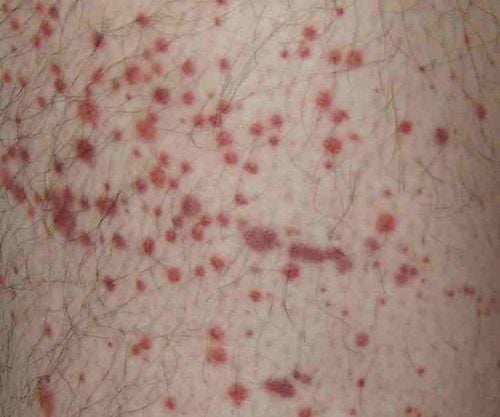
Gastrointestinal: Anorexia, nausea, vomiting, diarrhea, gastrointestinal bleeding. Abdominal pain, stomatitis, esophagitis, mucositis, dysphagia, constipation, weight loss, dehydration. Respiratory: Fludarabine also commonly causes dyspnea, rhinitis, pneumonia, cough, upper respiratory tract infections. Nosebleeds, sinusitis, pharyngitis, bronchitis, hemoptysis, allergic pneumonia, flu-like syndrome, hypoxia. Cardiovascular: Fludarabine often causes arrhythmia, paroxysmal tachycardia, congestive heart failure, chest pain, angina pectoris, myocardial infarction, phlebitis, deep embolism, aneurysm. Anemia, marrow failure, neutropenia, thrombocytopenia. Brain and nervous system: Fludarabine often causes cerebrovascular accident, ischemic stroke, cerebellar syndrome, headache, sleep disturbance, psychological damage, depression. Medullary dysplasia, acute myeloid leukemia, hemorrhage. Liver, kidney: Liver failure, liver dysfunction, gallstones. Dysuria, urinary retention, hematuria, renal dysfunction, renal failure, proteinuria. Muscles, bones, joints: Fludarabine also commonly causes myalgia, muscle weakness, paresthesia, arthralgia, back pain, osteoporosis. Body as a whole, other: Fatigue, fever, chills, pain, edema, rash. Peripheral edema, infection, sweating, decreased vision, hearing loss. Oily secretions on the skin, itching, rash, hair loss. Hyperglycemia and LDH. Hypersensitivity reactions, Herpes simplex infection, tumor lysis syndrome. If you see any strange symptoms after taking Fludarabine, you should immediately report it to your doctor or go to a medical facility to be checked.
4. Some notes when using Fludarabine medicine
Do not use Fludarabine in people with hypersensitivity to any of its components. Reduce dose or discontinue Fludarabine if serious side effects occur. During and after treatment with Fludarabine, hematological parameters should be checked regularly. During treatment with Fludarabine, if irradiated blood must be used, it should be used with caution. Test for uric acid and check blood levels of LDH before administering fludarabine in patients with large tumors because the drug increases the risk of tumor lysis syndrome. Dosage adjustment of fludarabine in patients with renal impairment. Monitor and closely monitor the use of Fludarabine in the elderly, patients with bone marrow failure. To limit the risk of infection, use of fludarabine with corticosteroids should be avoided. Both rapid and slow intravenous infusions of Fludarabine can cause neurotoxicity. Fludarabine should only be used in pregnant women if the potential benefit to the mother outweighs the risk of fetal toxicity and the condition is worsening and life-threatening for which there is no alternative medicine. . Women who are trying to become pregnant should not take the drug. Women who are taking the drug but become pregnant or are pregnant should be informed of the possible risk of death to the fetus. Breast-feeding women who wish to take Fludarabine should discontinue breast-feeding. Limit activities to drive or operate machinery while taking Fludarabine because the drug can cause visual disturbances, weakness, fatigue, convulsions, agitation, confusion. Fludarabine can interact with pentostatin and cause pulmonary toxicity, which can be fatal if taken concomitantly with pentostatin. Fludarabine inhibited the effect of the drug in people with leukemia when cytarabine was given first. In contrast, drug metabolism was stimulated when fludarabine was administered before cytarabine. Concomitant administration of fludarabine with live or inactivated vaccines, denosumab, natalizumab, trastuzumab, echinacea, pimecrolimus, imatinib, topical tacrolimus, sipuleucel-T, BCG, pentostatin, roflumilast, clozapine should be avoided because of the impaired effect or activity of the drug. influence (increase or decrease). Fludarabine is used to inhibit DNA synthesis in people with chronic lymphocytic leukemia when the disease is progressive or unresponsive to alkylating drugs.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.