This is an automatically translated article.
zanimex 500 oral tablet is used to treat mild to moderate infections of the lower respiratory tract caused by susceptible bacteria. So what is Zanimex? Let's learn the information about zanimex 500 in the following article to understand more about the effects and usage of zanimex 500.1. What is zanimex 500?
Zanimex has the main ingredient cefuroxime, a group of Cephalosporin antibiotics packaged in the form of 500mg blister-coated tablets. Cefuroxime Axetil is essentially a bactericidal agent that works by inhibiting bacterial cell wall synthesis. Cefuroxime Axetil acquires that activity in the presence of inhibition, in the presence of gram-negative and gram-positive bacteria, of several β-lactamases, including penicillinase and cephalosporinase. After binding to the penicillin-binding protein (PBP), Cefuroxime proceeds to inhibit bacterial cell wall synthesis. This leads to disruption of peptidoglycan (cell wall biosynthesis), which in turn interprets and causes bacterial cell death.
Bacterial resistance mechanism of zanimex 500 Bacteria resistant to cefuroxime antibiotics may be due to one or more of the following mechanisms
Hydrolysis by beta-lactamase; includes the inducible AmpC enzymes and the stabilizing expression of extended-spectrum beta-lactamases (ESBLs) in certain aerobic Gram-negative bacteria; Penicillin-binding proteins have reduced affinity for cefuroxime; The impermeability of the outer membrane, limiting the access of penicillin-binding proteins to cefuroxime in Gram-negative bacteria; Organisms already resistant to other parenteral cephalosporins are likely to be resistant to cefuroxime. Depending on the different resistance mechanisms acquired by penicillin-resistant organisms, resistance or susceptibility to cefuroxime may be reduced or reduced.
Absorption of zanimex 500 After oral administration Zanimex is absorbed from the gastrointestinal tract and rapidly hydrolyzed in the intestinal mucosa and in the blood to help release cefuroxime into the circulation. Use immediately after a meal for optimal absorption.
Following administration of Zanimex tablets, peak serum concentrations for the 500 mg dose of 7.0 mcg/ml occurred approximately 2 to 3 hours after dosing immediately after a meal. The rate of absorption of cefuroxime tablets is lower, resulting in a later peak serum concentration and also decreased systemic bioavailability (4 to 17% less). No accumulation of cefuroxime in the body occurred after repeated oral doses of 250 to 500 mg.
Distribution of the drug zanimex 500 Binding to plasma proteins is about 33%. In a study in 12 healthy volunteers following a single oral dose of cefuroxime axetil 500 mg tablets, the apparent volume of distribution was 50 L (CV% = 28%).
For common pathogens, concentrations of cefuroxime exceed the minimum inhibitory levels achievable in sinus tissue, bronchial mucosa, tonsils, bone, pleural fluid, interstitial fluid, synovial fluid, bile, phlegm and aqueous humor. When the meninges are inflamed, Cefuroxime crosses the blood-brain barrier.
Bioconversion of the drug zanimex 500 Cefuroxime is not metabolized.
Elimination of zanimex 500 The half-life of Zanimex in serum is from 1 to 1.5 hours. Cefuroxime is eliminated by glomerular filtration and tubular secretion. Renal clearance is in the range of 125 to 148 ml/min/1.73 m2.
Zanimex is excreted unchanged in the urine. In adults, within 12 hours about 50% of the dose is recovered in the urine. The pharmacokinetics of cefuroxime in pediatric subjects have not been studied.
In patients with renal impairment: In a clinical trial of 28 adults with normal renal function or with severe renal impairment (with creatinine clearance <30 mL/min), the elimination half-life was prolonged. related to the severity of renal failure. Therefore, the dosing interval should be extended in adult patients with creatinine clearance <30mL/min
The antibacterial activity of zanimex 500 Cefuroxime Axetil has been clinically demonstrated against most most of the following bacterial strains, both in clinical and in vitro infections.
Aerobic bacteria:
Gram-positive bacteria: Pneumococcus, Streptococcus pyogenes, Staphylococcus aureus (methicillin-susceptible isolates only) Staphylococcus saprophyticus (methicillin-susceptible isolates only), Staphylococcus epidermidis (methicillin-susceptible isolates only), Staphylococcus epidermidis (methicillin-susceptible isolates only) methicillin-susceptible isolates), Streptococcus agalactiae Gram-negative bacteria: Klebsiella pneumoniae a, Escherichia coli a, Haemophilus parainfluenzae, Haemophilus influenzae, Neisseria gonorrhoeae, Moraxella catarrhalis, Morganella morganii, Proteus inctans, Proteuscia mirtabilis, Providen. Most of the β-lactamase (ESBL) and extended-spectrum carbapenemase-producing isolates are resistant to Cefuroxime Axetil. Spirochetes, Borrelia burgdorferi
2. The effect of the drug zanimex 500
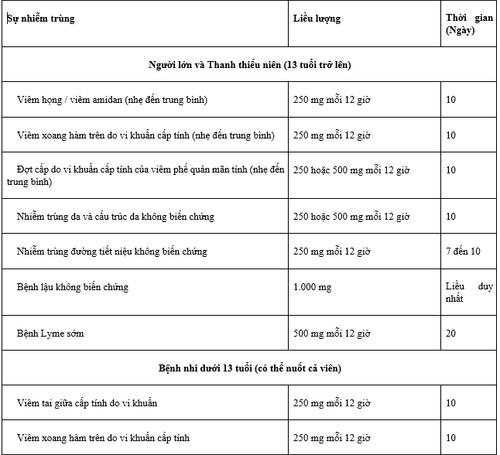
2.1. Indications for pharyngitis/tonsillitis Zanimex 500 tablets are indicated for the treatment of adult and pediatric patients (13 years of age and older) with mild to moderate pharyngitis/tonsillitis. caused by susceptible strains of Streptococcus pyogenes. However, the effectiveness of Zanimex 500 in the treatment of penicillin-resistant strains of Streptococcus pyogenes has not been demonstrated in clinical trials.
Treatment of otitis media Zanimex 500 Tablets are indicated for the treatment of pediatric patients (who can be swallowed whole) with acute bacterial otitis media caused by susceptible strains of Haemophilus influenzae (including strains of Haemophilus influenzae). β-lactamase-producing), Moraxella catarrhalis (including β-lactamase-producing strains), Streptococcus pneumoniae or Streptococcus pyogenes.
Maxillary Sinusitis Zanimex 500 Tablets are indicated for the treatment of adult and pediatric patients (13 years of age and older) with mild to moderate acute bacterial maxillary sinusitis caused by susceptible strains of Streptococcus pneumoniae or Haemophilus influenzae (only strains that do not produce β-lactamase).
Exacerbation of Acute Bacterial Chronic Bronchitis Zanimex 500 Tablets are indicated for the treatment of adult and pediatric patients (13 years of age and older) with acute bacterial exacerbations. mild to moderate chronic bronchitis, caused by susceptible strains of Streptococcus pneumoniae, Haemophilus influenzae (β-lactamase-negative strain), or Haemophilus parainfluenzae (β-lactamase-negative strain).
Uncomplicated Skin and Skin Structure Infections Zanimex 500 Tablets are indicated for the treatment of adult and pediatric patients (13 years of age and older) with uncomplicated skin and skin structure infections. caused by susceptible strains of Streptococcus pyogenes or Staphylococcus aureus (including β-lactamase-producing strains).
Uncomplicated Urinary Tract Infections Zanimex 500 Tablets are indicated for the treatment of adult and pediatric patients (13 years of age and older) with uncomplicated urinary tract infections caused by susceptible strains of caused by Escherichia coli or Klebsiella pneumoniae.
Uncomplicated Gonorrhea Zanimex 500 Tablets are indicated for the treatment of adult and pediatric patients (13 years of age and older) with uncomplicated gonorrhea.
Early Lyme Disease (erythema migratory) Zanimex 500 Tablets are indicated for the treatment of adult and pediatric patients (13 years of age and older) with early stage Lyme disease (erythema migratory) caused by susceptible strains of Borrelia burgdorferi.
2.2. Contraindications of zanimex 500 Zanimex 500 is contraindicated in patients with known hypersensitivity (eg, anaphylaxis) to Cefuroxime Axetil or to other β-lactam antibacterial agents (eg, penicillins and cephalosporins).
2.3. Undesirable effects of the drug zanimex 500 Blood and lymphatic system disorders: Hemolytic anemia, leukopenia, thrombocytopenia, thrombocytopenia. Gastrointestinal disorders: Pseudomembranous colitis Hepatobiliary disorders: Liver failure including hepatitis and cholestasis, jaundice. Immune system disorders: Anaphylaxis, serum sickness-like reactions. Nervous system disorders: Convulsions, encephalopathy. Skin and subcutaneous tissue disorders: Angioedema, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, urticaria. 2.4. Drug Interactions Zanimex reduces acid levels in the stomach. The drug may reduce the bioavailability of Zanimex 500 compared with fasting. Use of drugs that reduce gastric acidity may negate the effect of increasing the absorption of Zanimex 500 of food when taken in the postprandial state. Take Zanimex 500 at least 1 hour before or 2 hours after taking a short-acting antacid. Proton pump inhibitors and histamine-2 antagonists should be avoided.
Probenecid Co-administration of probenecid with Zanimex 500 tablets increases serum concentrations of cefuroxime. Concomitant use of probenecid with Zanimex 500 is not recommended.
Zanimex 500 may affect the intestinal microflora, leading to decreased estrogen reabsorption and decreased effectiveness of combined oral contraceptives. Concomitant use of Zanimex 500 with oral anticoagulants may increase the INR. Drug Interactions with Laboratory Tests False-positive reactions for glucose in the urine may occur with copper reduction tests (eg, Benedict's or Fehling's solution), but not with laboratory tests. Enzyme-based test for urinary tract. Because false-negative results can occur in the ferricyanide test, the glucose hexokinase or oxidase method should be used to determine blood or plasma glucose levels in patients receiving Zanimex 500. The presence of cefuroxime does not interfere with serum and urine creatinine testing by alkaline picrate method.
Drugs that reduce gastric acidity may result in lower bioavailability of cefuroxime axetil than in the fasted state and tend to cancel out the absorption-enhancing effect after eating.
2.5. Overdosage of zanimex 500 Overdose of cephalosporins can cause brain irritation leading to seizures or encephalopathy. Serum concentrations of cefuroxime can be reduced by hemodialysis and peritoneal dialysis.
3. How to use zanimex 500 effectively
Dosage in patients with impaired renal function Dose interval adjustment is required for patients with creatinine clearance less than 30 mL/min, as cefuroxime is eliminated primarily by the kidneys
| Độ thanh thải creatinin (mL / phút) | Liều dùng khuyến nghị |
| ≥30 | Không điều chỉnh liều lượng |
| 10 đến ˂30 | Liều cá nhân tiêu chuẩn được đưa ra sau mỗi 24 giờ |
| ˂10 (không chạy thận nhân tạo) | Liều cá nhân tiêu chuẩn được đưa ra sau mỗi 48 giờ |
| Chạy thận nhân tạo | Một liều tiêu chuẩn bổ sung duy nhất nên được tiêm vào cuối mỗi lần lọc máu |
Vinmec International General Hospital is one of the hospitals that not only ensures professional quality with a team of leading medical doctors, modern equipment and technology, but also stands out for its examination and consultation services. comprehensive and professional medical consultation and treatment; civilized, polite, safe and sterile medical examination and treatment space.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.













