This is an automatically translated article.
pH is a scale used to determine the acidity or base of a substance. Our bodies work extremely closely to control the pH of our blood and body fluids. Maintaining a balanced pH is extremely important for a healthy body.
1. What is the normal pH of blood?
The pH will be between 0 - 14. Neutral solutions, such as filtered water, will have a pH = 7
If pH < 7: Is acidic If pH > 7: Is basic. The normal pH of blood is between 7.35 and 7.45. This means that the blood will be slightly basic.
Usually gastric juice has a pH of about 3 - 5.5. A low pH will make it easier to digest food and kill bacteria in the stomach.
Độ pH thấp sẽ giúp tiêu diệt các vi khuẩn trong dạ dày dễ dàng hơn.
2. Assess acid-base status
To assess acid-base status we need to record the average values of the main parameters related to acid-base status and some basic terms as follows:
pH: Normal 7.35 – 7.45 PaCO2: Normal 36 - 44 mmHg Bicarbonate: Normal 22 - 26 mmol/L We need to be aware that these parameters will vary slightly between laboratories. Common terms used to describe acid-base conditions include:
Acidosis: Indicated when blood pH is low, < 7.35. Alkalinity: Indicated when blood pH is high, > 7.45. Acidosis: Indicated by any process that, if left unchecked, leads to acidosis. This problem can occur through one of two mechanisms: Respiratory acidosis exists when PCO2 is high (>44) and metabolic acidosis exists when HCO3- is low (<22). Alkalosis: Indicated by any process that, if left unchecked, leads to alkalosis. This problem can occur through one of two mechanisms: Respiratory alkalosis exists when PCO2 is low (< 36 ) and metabolic alkalosis exists when HCO3- is high (> 26).
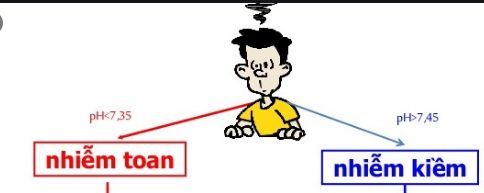
Dựa vào chỉ số pH máu có thể đánh giá tình trạng toan máu – kiềm máu
3. Steps to read arterial blood gas
Steps to take arterial blood gas readings to assess acid-base status:
View pH and compare with normal range Identify early processes leading to pH change Calculate blood anion gap Determine progress compensatory process when it exists Detects the existence of other diseases or has a combined acid-base course
4. Causes of blood pH changes
4.1 Increase blood pH
There are many causes of increased blood pH. Having a medical condition can temporarily raise blood pH. Certain foods can also raise blood pH.
4.1.1 Dehydration
Dehydration of the body can increase the pH of the blood. This is because when you lose water, you also lose electrolytes (like salts and minerals like sodium and potassium). Causes of dehydration include: Sweating, vomiting, diarrhea
Use of diuretics and other medications can also make you urinate a lot and lead to an increase in blood pH. Treatment for dehydration includes drinking plenty of fluids and replacing electrolytes. Sports drinks can also sometimes help with electrolyte replacement.

Cơ thể bị mất nước có thể làm tăng độ pH của máu
4.1.2 Kidney problems
The kidneys help the body maintain acid and base balance. Kidney problems can lead to an increase in blood pH. This is because the kidneys will no longer be able to remove alkaline substances from the urine and may return them to the blood, such as bicarbonate.Use of medications and other treatments can also help lower blood pH.
4.2 Lowering blood pH
Acidosis can affect the functions of all organs in the body.
Certain health problems can cause acid levels to rise in the blood. Acids that can lower blood pH include: Lactic Acid, Keto Acid, Sulfuric Acid, Phosphoric Acid, Hydrochloric Acid, Carbonic Acid.
4.3 Diet
An unbalanced diet can temporarily lower blood pH. Eating too little or going far without eating for a long time can make your blood more acidic. Avoid foods that cause acid in the body including: milk (cow's milk, cheese, yogurt), poultry, eggs, pork, fish, grains (wheat, bread, rice). ..).
Maintain your body's pH by eating more alkaline foods, including fresh and cooked vegetables, and fruits. Avoid fad diets or binge eating.

Bổ sung nhiều ray xanh giúp duy trì độ pH trong cơ thể
4.4 Diabetic acidosis
If you have diabetes, your blood may become more acidic if your blood sugar is not well controlled. Diabetic acidosis occurs when the body doesn't make enough insulin or doesn't use insulin effectively.
Insulin helps move sugar from food into cells to be burned for energy.
If insulin is not used, the body will begin to break down stored fat for energy. This will produce a waste product called ketones and cause low blood pH.
Call 911 right away if your blood sugar rises above 300mg/dl, or 16mmol/L. Or have the following symptoms: Excessive thirst, frequent urination, fatigue, weakness, nausea or vomiting, difficulty breathing, bad breath, abdominal pain, loss of consciousness

Nhiễm toan do tiểu đường sẽ xảy ra khi cơ thể không tạo ra đủ insulin
4.5 Metabolic acidosis
Low blood pH due to kidney disease or kidney failure is called metabolic acidosis. This condition occurs when the kidneys are not working effectively to remove acid from the body and will increase the acid level in the blood and lower the blood pH.
Symptoms of metabolic acidosis include: Fatigue and weakness, loss of appetite, nausea and vomiting, headache, heart palpitations, shortness of breath.
Treatment of metabolic acidosis involves taking medications to help the kidneys work better. In severe cases, dialysis or a kidney transplant may be possible.
4.6 Respiratory acidosis
When the lungs are not able to remove carbon dioxide from the body quickly enough, the blood pH drops, a condition known as respiratory acidosis. Respiratory acidosis can occur when you have chronic lung disease or severe lung disease.
People who have undergone bariatric surgery or abuse sleeping pills or opioids are at risk for respiratory acidosis.
Vinmec International General Hospital is one of the hospitals that not only ensures professional quality with a team of leading medical doctors, modern equipment and technology, but also stands out for its examination and consultation services. comprehensive and professional medical consultation and treatment; civilized, polite, safe and sterile medical examination and treatment space. Customers when choosing to perform tests here can be completely assured of the accuracy of test results.
Customers can directly go to Vinmec Health system nationwide to visit or contact the hotline here for support.
SEE MORE
Meaning of blood and urine tests in the general health checkup package The role of determining blood group before blood transfusion Meaning of indicators in blood tests