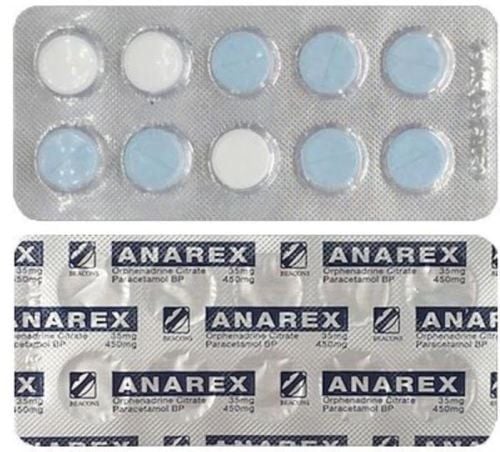
Cataflam 25mg is a remedy for chronic arthritis and some short-term symptoms such as acute attacks of peripheral arthritis and gouty arthritis.
1. What is Cataflam 25mg?
Cataflam contains the active ingredient Diclofenac potassium, belonging to the group of pain relievers. It is formulated as 25mg sugar-coated tablets and manufactured by Interpharma Manufacturing Company Limited.
Cataflam 25 mg is prescribed by doctors for the following cases:
- Treating pain, inflammation, and swelling after injuries such as spra
- Relieving pain after surgery or minor surgery, especially inflammation and swelling after orthopedic surgery or dental surgery.
- Improving pain or inflammation in gynecology such as adnexitis or primary dysmenorrhea.
- Acute musculoskeletal disorders and injuries such as periarthritis (especially frozen shoulder), tendinitis, tenosynovitis, bursitis, sprains, strains, and dislocations.
- Reducing migraine attacks.
- Spinal pain syndrome.
- Rheumatoid arthritis, osteoarthritis.
- Adjunctive therapy in severe inflammatory pain infections of the nose, ear, or throat such as tonsillitis, pharyngitis, otitis.
2. What are uses of Cataflam 25mg
2.1 Pharmacodynamics
Cataflam sugar-coated tablets contain diclofenac potassium, a non-steroidal compound with pronounced analgesic, anti-inflammatory, and antipyretic properties that have been clinically proven.
The mechanism of action of diclofenac potassium tablets, like other NSAIDs, is not fully understood but is related to the inhibition of cyclooxygenase (COX-1 and COX-2).
Diclofenac is a potent inhibitor of prostaglandin synthesis in vitro. The concentrations of diclofenac achieved during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and enhance the action of bradykinin in producing pain in animal models. Prostaglandins are mediators of inflammation. Because diclofenac is an inhibitor of prostaglandin synthesis, its mode of action may be due to a reduction in prostaglandins in peripheral tissues.
Cataflam tablets have a rapid onset of action and are therefore suitable for the treatment of acute pain and inflammation.
In migraine attacks, Cataflam sugar-coated tablets have been shown to be effective in reducing headache and improving associated nausea.
Diclofenac in vitro does not inhibit proteoglycan synthesis in cartilage at concentrations comparable to those achieved in humans.
2.2. Pharmacokinetics
Absorption:
- Diclofenac is rapidly and completely absorbed from film-coated tablets. Food intake does not affect drug absorption.
- Peak plasma concentrations after a 50 mg film-coated tablet are 3.9 μmol/L after 20-60 minutes. Plasma concentrations show a linear relationship with dose size.
- Diclofenac undergoes first-pass metabolism and is extensively metabolized.
Distribution:
- Diclofenac is highly bound to plasma proteins (99.7%), mainly albumin (99.4%).
- Diclofenac has been detected at low concentrations (100 ng/mL) in breast milk in a lactating mother. The estimated amount ingested by a breastfed infant is equivalent to a dose of 0.03 mg/kg/day.
Elimination:
- The total body clearance of diclofenac in plasma is 263 ± 56 mL/min (mean ± SD).
- The terminal half-life in plasma is 1-2 hours.
- Repeated administration of diclofenac potassium tablets at a daily dose of 50 mg t.i.d. for 8 days does not lead to accumulation of diclofenac in plasma.
- Approximately 60% of the dose is excreted in the urine as metabolites, and less than 1% unchanged. The remainder of the dose is eliminated as metabolites in the bile via the feces.
3. Contraindications of Cataflam 25mg
- Patients with hypersensitivity to the active substance or to any of the excipients of Cataflam 25 mg
- Patients with active peptic ulcer or intestinal perforation
- Last trimester of pregnancy
- Patients with severe hepatic impairment, severe renal impairment (GFR < 15 mL/min/1.73m2)
- Patients with congestive heart failure (NYHA class II to IV), peripheral arterial disease, ischemic heart disease, cerebrovascular disease
- Patients with asthma, urticaria, or acute rhinitis triggered by acetylsalicylic acid or other NSAIDs
- History of gastrointestinal bleeding or perforation related to NSAID therapy.
4. Side effects of Cataflam 25mg
Side effects on the central nervous system:
- Uncommon: Dizziness, vertigo, headache
- Rare: Somnolence
- Very rare: Tremor, agitation, depression, convulsions, insomnia, memory impairment, sensory disturbances, convulsions,...
Side effects on gastrointestinal system:
- Uncommon: Nausea, epigastric pain, abdominal distension, anorexia, intestinal spasms, diarrhea
- Rare: Peptic ulcer, gastrointestinal bleeding
- Very rare: Glossitis, stomatitis, constipation, pancreatitis, Crohn's disease, nonspecific ulcerative colitis, stricture…
Side effects on skin:
- Uncommon: Skin rash, itchy urticaria
- Rare: Eczema, Stevens-Johnson syndrome, bullous exanthema, erythema multiforme, exfoliative dermatitis, Lyell's syndrome, alopecia, allergic purpura, photosensitivity
Side effects on sensory organs:
- Hearing impairment
- Taste disturbances
- Visual disturbances (diplopia, blurred vision)
- Sensory side effects are very rare with Cataflam.
Side effects on kidneys (rare):
- Edema
- Acute renal failure
- Papillary necrosis
- Nephrotic syndrome
- Interstitial nephritis
Side effects on the liver:
- Acute hepatitis
- Hepatitis with/without jaundice
- Increased blood aminotransferases
Other side effects:
- Leukopenia
- Aplastic anemia
- Thrombocytopenia
- Agranulocytosis
- Hypersensitivity (pneumonitis, vasculitis, asthma)
- Hypertension
- Congestive heart failure
- Palpitations
5. Drug interactions
Lithium: By using concomitantly, Cataflam may increase plasma lithium levels. Lithium levels in serum should be monitored.
Digoxin: By using concomitantly, Cataflam may increase plasma digoxin levels. Monitoring digoxin levels in serum is recommended.
Diuretics and antihypertensive drugs: As with other NSAIDs, concomitant use of diclofenac with diuretics and antihypertensive drugs (for example: beta-blockers, ACE inhibitors may reduce the antihypertensive effect due to inhibition of vasodilating prostaglandin synthesis).
Therefore, the combination should be administered with caution, and patients, especially the elderly, should have their blood pressure monitored regularly. Patients should be adequately hydrated and consideration should be given to monitoring renal function after initiating concomitant therapy, and periodically thereafter, especially in patients receiving diuretics and ACE inhibitors, since the risk of nephrotoxicity is increased.
Drugs known to cause hyperkalemia: Concomitant treatment with potassium-sparing diuretics, ciclosporin, tacrolimus, or trimethoprim may increase serum potassium levels, and therefore should be monitored regularly.
Anticoagulants and antiplatelet agents: Caution should be exercised because concomitant use may increase the risk of bleeding. Although clinical studies have not shown that diclofenac affects the effects of anticoagulants, there have been reports of an increased risk of bleeding in patients taking Cataflam concomitantly with anticoagulants. Therefore, to ensure that the dosage of anticoagulants is not altered, close monitoring of such patients is necessary. Like other nonsteroidal anti-inflammatory drugs, diclofenac at high doses can reversibly inhibit platelet aggregation.
Other NSAIDs including selective cyclooxygenase-2 inhibitors and corticosteroids: Concomitant use of Cataflam with other systemic NSAIDs or corticosteroids may increase the risk of gastrointestinal ulceration or bleeding. Therefore, the concomitant use of two or more NSAIDs should be avoided.
Selective serotonin reuptake inhibitors (SSRIs): Concomitant use of SSRIs may increase the risk of gastrointestinal bleeding.
Antidiabetic drugs: Clinical studies have shown that diclofenac can be used with oral antidiabetic drugs without affecting the clinical efficacy of the drugs. However, there have been isolated reports of both hypoglycemic and hyperglycemic effects requiring adjustment of the antidiabetic drug dosage during treatment with Cataflam. For this reason, blood glucose levels should be monitored as a precautionary measure during concomitant therapy.
Methotrexate: Cataflam may inhibit the renal clearance of methotrexate and thus increase methotrexate concentrations. Caution should be exercised when using NSAIDs, including diclofenac, less than 24 hours before treatment with methotrexate.
Ciclosporin: Diclofenac in Cataflam, like other NSAIDs, may enhance the nephrotoxicity of ciclosporin due to effects on renal prostaglandins. Therefore, lower doses than those used in patients not taking ciclosporin should be used.
Tacrolimus: There may be an increased risk of nephrotoxicity when NSAIDs are administered concomitantly with tacrolimus. This may be mediated by the antiprostaglandin effect on the kidney of both the NSAID and the calcineurin inhibitor.
Quinolone antibodies: Convulsions may occur due to an interaction between quinolones and NSAIDs. This may occur in patients with or without a previous history of convulsions or seizures. Therefore, caution should be exercised when considering the use of quinolones in patients currently on NSAID therapy.
Phenytoin: When phenytoin is used concomitantly with Cataflam, the plasma concentration of phenytoin should be monitored due to the expected increase in phenytoin exposure.
Colestipol and cholestyramine: These agents may delay or decrease the absorption of Cataflam. Therefore, Cataflam should be taken at least one hour before or 4 to 6 hours after administration of colestipol/cholestyramine.
Cardiac glycosides: Concomitant use of cardiac glycosides and Cataflam in patients may exacerbate heart failure, increase plasma glycoside levels, and reduce GFR.
Mifepristone: NSAIDs should not be used for 8-12 days after administration of mifepristone because these drugs may reduce the effect of mifepristone.
Strong CYP2C9 inhibitors: Caution is advised when co-prescribing Cataflam with strong CYP2C9 inhibitors (such as voriconazole), which may significantly increase peak plasma concentrations and exposure to diclofenac due to inhibition of diclofenac metabolism.
Zidovudine: Increased risk of haematological toxicity when NSAIDs are used with zidovudine. There is evidence of an increased risk of bleeding and haematoma in HIV (+) haemophiliacs treated concomitantly with zidovudine and ibuprofen.
6. Precautions when using Cataflam 25mg
Cataflam is generally well-tolerated when used at the recommended dosage for short periods. However, in patients with a history of peptic ulcer, liver disease, or Crohn's disease, close monitoring is necessary to avoid the risk of gastrointestinal bleeding.
Cataflam is metabolized by the liver and may increase abnormal liver enzymes. In addition, this menstrual pain reliever can trigger attacks in patients with porphyrin metabolism disorders.
Caution is required when using the drug in the elderly, patients with heart failure, and renal impairment. In addition, Cataflam has the ability to inhibit platelet aggregation, so caution should be exercised in patients with bleeding disorders.
Cataflam may help reduce the symptoms of infection (fever, pain, and inflammation). Therefore, in cases of suspected pelvic inflammatory disease, an examination should be performed before use.
If signs of gastrointestinal bleeding or perforation are noticed (vomiting coffee-ground emesis, bloody stools, severe epigastric pain), stop the drug and seek medical attention immediately.
Concomitant use of Cataflam with alcohol can increase the risk of stomach ulcers and gastrointestinal bleeding. In addition, during the use of the drug, you should limit the consumption of foods that are harmful to the stomach such as carbonated drinks, sour, salty, and spicy foods.
Some patients may experience visual disturbances while taking the drug. In this case, it is necessary to limit the use of vehicles or machinery.
7. How to use Cataflam 25mg effectively
Instruction:
- Cataflam is taken orally. It can be taken with or after meals as clinical trials have shown that food does not reduce the extent of drug absorption in the body. However, in cases where patients do not have stomach pain, the drug can be taken before meals so that the drug can work in the shortest time.
The usual dosage for adults:
- Initial daily dose: 100-150mg/day
- Should be divided into 2-3 doses, taken 4 hours apart
In cases of mild pain or use for children over 14 years old:
- Use 75-100mg/day
- Divided into 2-3 doses per day
Dosage for the treatment of primary dysmenorrhea:
- Use 50-150mg/day
- In special cases, 200mg/day can be used
- Should be divided into 3 doses per day
Dosage for the treatment of migraine attacks:
- Initial dose: 50mg/dose
- After 2 hours if symptoms do not improve, an additional 50mg/dose can be taken
- Then continue taking 50mg for 4-6 hours
- Maximum dose: 200mg/24 hours
In conclusion, Cataflam 25mg is used to treat chronic arthritis. Patients should read the instructions carefully and consult their doctor before use. Absolutely do not self-medicate as you may experience unwanted side effects.
Please call HOTLINE or make your reservation directly HERE to arrange an appointment. You may also download the MyVinmec app to schedule appointments faster and conveniently manage your reservations.