This is an automatically translated article.
Voritab - 50 is an imported product, indicated for the treatment of patients with Candida blood infections and invasive Candida infections very effectively. To ensure the effectiveness of use when using Voritab - 50, users need to follow the instructions of their doctors and refer to the following article for more information about the uses of Voritab - 50.
1. What does Voritab - 50 do?
1.1. What is Voritab - 50? Voritab - 50 medicine contains the following ingredients and excipients: Voriconazole 200 mg, Lactose, povidone, corn starch, magnesium stearate, purified talc, colloidal anhydrous silica, polacribin potassium (tulsion 339), white opadry 58901 and macrogol 6000.
Voritab - 50 is manufactured by Synmedic Laboratories, and the drug has been licensed for circulation in Vietnam with registration number: VN2-370-15.
Dosage form: Tablets.
1.2. What does Voritab - 50 do? Voritab - 50 drug is effective in:
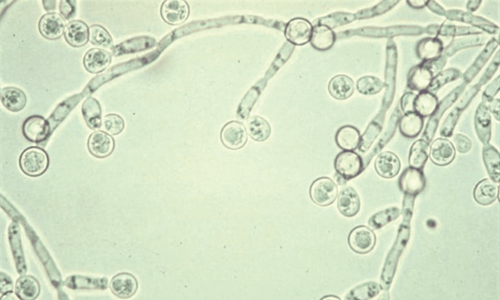
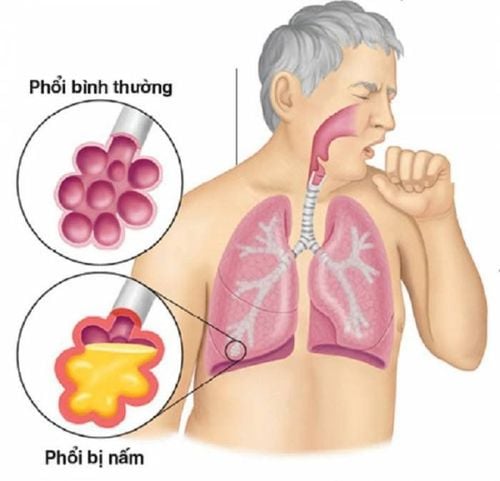
Used to treat patients with invasive Aspergillus infections (such as fungal infections first in the lungs and then spreading to other organs by blood). Used to treat candidaemia in patients without neutropenia and disseminated candidiasis of the skin, abdomen, kidneys, bladder wall, and wounds. Used to treat patients with severe invasive candidiasis in cases of resistance to fluconazole (including c. krusei). Used to treat severe fungal infections caused by Scedosporium spp. and Fusarium spp. , or used for patients who have not responded to other treatments.
2. Usage of Voritab - 50
2.1. How to take Voritab - 50 Patients should take Voritab - 50 before or after eating at least 1 hour. 2.2. Dosage of Voritab - 50 Dosage of Voritab - 50 for people 13 years of age and older:
Dosage for children 2 -12 years old:
For patients with pharyngeal candidiasis: The duration of drug treatment is from 7 to 14 days until the symptoms are gone.
Adjust dose:
If the patient has not responded adequately, the maintenance dose can be increased to 300mg x 2 times/day. If the patient does not improve with this dose, reduce to 200mg x 2 times/day for patients over 40kg and 100kg x 2 times a day for patients under 40kg. Elderly: No dose adjustment is required for the elderly. Patients with renal impairment: No dose adjustment is required for patients with renal impairment. Patients with hepatic impairment: Adjust the dose by half for patients with mild and moderate hepatic impairment. For patients with severe liver failure, it is necessary to consult a specialist, only for people with severe liver failure when the benefits outweigh the risks and the patient must be monitored to avoid drug toxicity. Handling missed dose:
If the patient forgets to take a dose, take it immediately. In case the patient is close to the time to take the next dose, just take the next dose as usual, do not combine the missed dose. Overdose treatment:
If the patient uses the drug in an overdose, there will be some common symptoms such as photophobia. Therefore, patients need to be treated symptomatically and supportively.
3. Note when using Voritab - 50
Patients with hypersensitivity to the ingredients of the drug: Caution should be exercised in patients who have ever been hypersensitivity to any other azole antifungals (itraconazole, ketoconazole..) or who have ever had any allergies. Cardiovascular Patients: QT prolongation has been shown to be associated with some azole antifungals, including voriconazole. Therefore, caution should be exercised in people at high risk such as: history of chemotherapy for heart disease, cardiomyopathy, potassium deficiency, taking drugs with synergistic effects. And caution when using this drug for patients at risk of arrhythmia such as: cardiomyopathy, especially in cases with heart failure, sinus bradycardia, arrhythmia symptoms, concomitant use with traction drugs QT interval length. Electrolyte disturbances include: Potassium depletion, magnesium depletion, calcium depletion, if necessary monitoring and correction before, during treatment with voriconazole. Hepatotoxicity: In clinical studies, severe hepatic reactions (including: hepatitis, cholestatic and sudden hepatic failure, including death) have been reported in clinical studies. Hepatic reactions occur mainly in patients with serious illness (malignant liver disease). Temporary hepatic reactions (hepatitis and jaundice) may occur in patients without risk factors. Liver function is restored upon discontinuation of the drug. Visual effects: Visual effects have been reported: blurred vision, optic neuritis and papilledema. Renal Effects: Acute renal failure has been reported in critically ill patients. In patients receiving voriconazole concomitantly with medicinal products known to impair renal function, renal function should be monitored, especially serum creatinine. bar. Skin effects: Exfoliative dermatitis, Steven-Johnson syndrome, has occurred but rarely. If a skin rash is observed, close monitoring is required and the drug is discontinued if the disease progresses. Use in children: The safety and efficacy of voriconazole in children under 2 years of age have not been established. For children over 2 years old only. Liver function tests are required for both children and adults. Bioavailability may be limited in children 2 to 12 years of age with malabsorption or low birth weight, in whom intravenous administration is required. Voritab - 50 tablets contain lactose and should therefore be used with caution in people with lactose intolerance, Lapp lactase deficiency, or lactose-galactose malabsorption. Voriconazole can cause blurred vision, visual changes, and photophobia, so patients should avoid dangerous jobs such as driving or operating machines while taking voriconazole. for pregnant women. Women of childbearing age, if treated with voriconazole, must use safe contraception. The excretion of voriconazole in breast milk has not been investigated. Use only for nursing mothers when absolutely necessary and the benefits outweigh the risks
4. Voritab - 50 . side effects
The most common side effects of Voritab - 50 include: Peripheral edema, headache, visual disturbances (blurred vision, photophobia, abdominal pain, nausea, vomiting, diarrhea, rash) , fever, increased ASAT, ALAT, alkaline phosphatase, GGT, LDH, bilirubin, serum creatinine, decreased red blood cells, myelosuppression, leukopenia, thrombocytopenia, purpura, anemia, dizziness, smearing, tremor sedation, agitation, paresthesia, acute respiratory distress syndrome, pulmonary edema, chest pain and back pain Less common side effects of Voritab - 50 are: The patient has a longer QT interval, increased blood nitrogen, Hypercholesterolemia, ventricular fibrillation, ventricular arrhythmias, syncope, tachycardia or bradycardia and coagulation disorders Rare side effects of Voritab - 50 are: Torsade de pointes, atrioventricular block, nodal arrhythmias. Atrioventricular, convulsive and encephalopathy If experiencing these symptoms, the patient should stop using Voritab - 50 and notify the doctor for appropriate management.
5. Voritab drug interactions - 50
The interaction of Voritab - 50 with other agents can reduce the effectiveness of the drug or increase the risk of side effects for users. Therefore, patients need to consult a pharmacist, a specialist when they want to use it at the same time with other drugs.
6. How to store Voritab - 50
Voritab medicine should be stored at a temperature below 30°C, away from moisture and direct light.
Above is all information about Voritab, patients need to carefully read the instructions for use, consult a doctor / pharmacist before using. Absolutely do not arbitrarily buy Voritab medicine at home because it may encounter unwanted side effects.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.