This is an automatically translated article.
Stomex medicine is indicated in the treatment of peptic ulcers, gastroesophageal reflux disease. So how to use Stomex? What precautions should be taken when using this drug? Let's find out the necessary information about Stomex drug through the article below.
1. Uses of Stomex
1.1 Indications Stomex is indicated for use in the following cases:
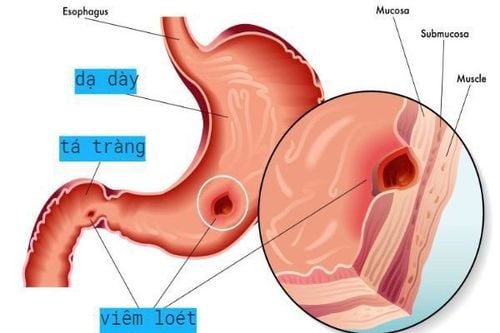
Severe gastritis Gastrointestinal ulcers including gastric and duodenal ulcers , Reflux esophagitis Zollinger-Ellison syndrome (ZES) Disorders other digestion due to acid. 1.2 How to use - Dosage How to use:
Stomex is used orally.
Dosage:
Duodenal ulcer, gastric ulcer and reflux esophagitis: 20 mg once daily at breakfast, used for 2 weeks, if necessary can be used for another 2 weeks.
Intractable ulcers: 40 mg, once daily for 4 - 8 weeks.
Zollinger - Ellison syndrome: Start 60 mg once daily and then adjust dose according to response. Doses above 80 mg daily should be divided into 2 doses.
Note: The above dosage is for reference only. The specific dose depends on the condition and the progression of the disease. To get the right dose, you need to consult your doctor or healthcare professional.
1.3 Overdose, missed dose and management Overdose:
There is no information on effects in humans in case of overdose. Oral doses up to 160 mg are well tolerated.
Missed dose:
If you miss a dose, take it as soon as you remember. However, if it is almost time for your next dose, skip the missed dose and take your next dose at the scheduled time. Note that double the prescribed dose should not be taken.
2. Side effects of the drug Stomex
Common:
Headache, drowsiness, dizziness. Nausea, vomiting, abdominal pain, diarrhea, constipation, abdominal distension. Uncommon:
Insomnia, confusion, dizziness, fatigue. Urticaria, itching, rash. Increased transaminases (reversible). Rare:
Sweating, peripheral edema, hypersensitivity including angioedema, fever and anaphylaxis. Leukopenia, thrombocytopenia, reduction in whole blood cells, agranulocytosis. Reversible confusion, agitation, depression, hallucinations in elderly patients and especially in critically ill patients, hearing disorders. Large breasts in men. Gastritis, Candida infection, dry mouth. Jaundice or non-jaundice hepatitis, encephalopathy in people with liver failure. Bronchospasm. Joint pain, muscle pain. Interstitial nephritis. Before using the drug, you should carefully read the instructions for use and refer to the information below.
3. Notes when using Stomex
Contraindications
Stomex is contraindicated in the following cases:
Pregnancy and lactation. Patients with hypersensitivity to any ingredient of the drug. Note:
If the patient has symptoms such as a lot of weight loss, prolonged vomiting, difficulty swallowing, vomiting blood or black stools), suspected or is having a stomach ulcer, the possibility of malignancy should be excluded. because treatment can alleviate symptoms and delay diagnosis. The combination of proton pump inhibitors (PPIs) and atazanavir is not recommended. If coadministration of PPIs and atazanavir is deemed necessary, close monitoring of clinical manifestations (e.g. viral infection) is required in combination with increasing the dose of atazanavir to 400 mg and 100 mg of ritonavir, the dose of omeprazole should not be increased. As with other acid blockers, omeprazole may decrease the absorption of vitamin B12 (cyanocobalamin) due to a decrease or lack of hydrochloric acid. Therefore, this factor should be considered in patients with reduced body stores or at risk of reduced vitamin B12 absorption if treated for a long time. Omeprazole is an inhibitor of CYP2C19. When starting or ending treatment with omeprazole, the risk of drug interactions with drugs metabolised by CYP2C19 should be taken into account. When monitoring drug interactions between clopidogrel and omeprazole, the clinical correlation of this interaction is not clear. As a precaution, however, clopidogrel and omeprazole should not be used concurrently. Severe hypomagnesaemia has been reported in patients treated with PPIs for at least 3 months and in most cases for about 1 year. Symptoms of severe hypomagnesaemia may occur, such as fatigue, muscle spasticity, delirium, convulsions, dizziness, and ventricular arrhythmias, but these symptoms may be insidious and go unnoticed. In the majority of patients with hypomagnesaemia, the condition improved after magnesium supplementation and discontinuation of PPIs. It is advisable to measure magnesium levels before initiating therapy and periodically during treatment in patients requiring long-term therapy or requiring concomitant PPIs with digoxin or other hypomagnesaemic agents (e.g. diuretics). urine). Use of PPIs, especially at high doses and for a long time (> 1 year), may slightly increase the risk of hip, wrist, and spine fractures, mainly in the elderly or in the presence of other medical conditions. other risk factors. Observational studies have shown that PPIs increase the overall risk of fracture by 10 to 40%, in some cases possibly due to other factors. Patients at risk for osteoporosis should be cared for according to currently available clinical guidelines and should be adequately supplemented with vitamin D and calcium. Pregnant women: Although omeprazole has not been found in experimental studies to cause malformations and toxicity to the fetus, it should not be used in pregnant women, especially in the first 3 months. Lactation: The use of omeprazole in nursing mothers is not recommended. Omeprazole has virtually no effect on the ability to drive or use machines. Undesirable effects such as dizziness and visual disturbances may occur. At that time, the patient should not drive or operate machinery.
4. Drug interactions
Drugs whose bioavailability is dependent on gastric pH
Due to its inhibitory effect on gastric acid secretion, omeprazole could theoretically affect the absorption of some drugs whose bioavailability is mainly dependent on gastric pH. dependent on gastric pH (eg: Ketoconazole, ampicillin, and iron salts).
Drugs metabolized by cytochrome P450 (CYP)
Omeprazole may prolong the excretion time of diazepam, warfarin and phenytoin, which are metabolized by oxidation in the liver.
Although interactions with theophylline or propranolol are generally unknown, there have been clinical studies of interactions with drugs metabolized by the cytochrome P450 system (eg: Cyclosporine, disulfiram, benzodiazepines). Patients should be monitored so that, if necessary, the dosage of these drugs should be adjusted when co-administered with Stomex.
Indications for a combination of omeprazole and viriconazole (a combination that inhibits both CYP2C19 and CYP3A4) results in double the results when omeprazole is used alone.
Antiviral
Omeprazole has been reported to interact with some antiviral drugs. The clinical importance and mechanism behind these interactions are unknown.
Antibiotics
Omeprazole 40 mg has been used in combination with clarithromycin 500 mg every 8 hours in healthy men. Steady-state plasma concentrations of omeprazole are increased in combination with clarithromycin.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.