This is an automatically translated article.
Rodogyl is a combination of two antibiotics, spiramycin and metronidazole, commonly used in the treatment and prevention of oral infections. Because this is a combination of 2 ingredients, it is easy for patients to confuse the content. The following article will provide readers with information about Rodogyl drug concentrations, dosage and some notes when using Rodogyl.1. What are the strengths of Rodogyl?
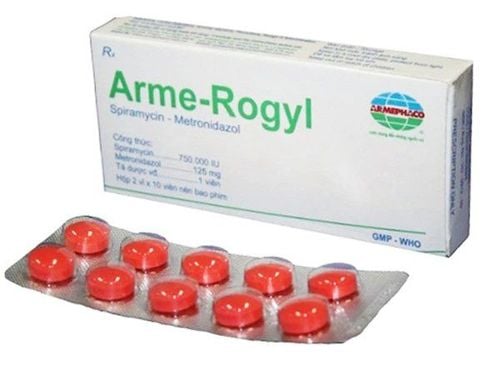
Rodogyl drug has the main active ingredients spiramycin and metronidazole. A combination drug of spiramycin, an antibiotic of the macrolide group, and metronidazole, a nitro-5-imidazole antibiotic for the treatment of mainly oral infections. Therefore, Rodogyl is also known by many as Rodogyl oral antibiotic. Many patients still have questions about the content of Rodogyl. Some people say that Rodogyl is 125mg, others think that Rodogyl is 750mg. In fact, Rodogyl has two main active ingredients, spiramycin with a concentration of 750 000IU and metronidazole 125 mg. Indications of Rodogyl include acute, chronic or recurrent oral sinus infections, specifically:
Tooth abscess Inflammation of the mandible subcutaneous tissue Inflammation of the jaw, gingivitis, periodontitis Periodontitis, inflammation of the gland. parotid foam or inflammation of the submandibular salivary glands. Prophylaxis of infection after oral surgery.
2. Dosage of Rodogyl
2.1 Treatment of infections Adults:
Take 4 to 6 Rodogyl tablets per day (approximately 3 to 4.5 million IU of spiramycin and 500 to 750mg of metronidazole), divided 2-3 times a day and taken with meals. For severe cases, the dose of Rodogyl can be increased up to 8 tablets a day.
Children:
From 6 to 10 years old: take 2 Rodogyl tablets per day (approximately 1.5 million IU of spiramycin and 250mg of metronidazole). 10 to 15 years: take 3 Rodogyl tablets per day (approximately 2.25 million IU of spiramycin and 375 mg of metronidazole). 2.2 Prophylaxis of local infections after oral surgery: Adults: take 4 to 6 Rodogyl tablets per day, divided into 2-3 times and take with meals.
Children:
From 6 to 10 years old: the dose is 2 Rodogyl tablets per day (about 1.5 million IU of spiramycin and 250mg of metronidazole). 10 to 15 years: 3 Rodogyl tablets per day (approximately 2.25 million IU of spiramycin and 375 mg of metronidazole).
3. What are the contraindications of Rodogyl?
Rodogyl is contraindicated in the following cases:
Hypersensitivity to imidazole, spiramycin or any of the excipients in the composition Children under 6 years old because the tablet dosage form is not suitable for this audience.
4. Undesirable effects when using Rodogyl
4.1 Side effects of spiramycin: Digestive system: Abdominal pain, nausea, vomiting, diarrhea and some rare cases of pseudomembranous colitis. Skin: Rash, pruritus, urticaria, Quincke's edema, anaphylaxis (very rare). Acute systemic purulent erythema (very rare) Central and Peripheral Nervous System: Occasionally, patients may experience transient paresthesias. Liver symptoms: Effects on liver function test results (rare). Hematologic: Some cases of hemolytic anemia have been reported with spiramycin (very rare) 4.2 Side effects of metronidazole Gastrointestinal system: Common gastrointestinal disturbances such as epigastric pain, nausea, vomiting, diarrhea. Glossitis, dry mouth feeling, stomatitis, metallic taste in mouth, loss of appetite. Pancreatitis reversible on discontinuation of treatment (very rare) Skin: Hot flashes, itching, rash, sometimes illness patient with fever. Urticaria, Quincke's edema or, rarely, anaphylaxis. Central and Peripheral Nervous System: Headache, Peripheral Neuropathy, Convulsions, Dizziness. Psychiatric disorders: Confusion, hallucinations. Hematological Effects: Very rarely patients have neutropenia, agranulocytosis and thrombocytopenia. Hepatic symptoms: In rare cases, patients may develop reversible liver dysfunction and obstructive hepatitis. Other effects may include: Red-brown urine due to water-soluble pigments due to drug metabolism.
5. Notes when using the drug Rodogyl
Avoid taking the drug with alcoholic beverages, because it can cause a reaction similar to d isulfiram . If the patient is suspected of having systemic pustular erythema (generalized erythema, fever associated with pustules occurring at the start of treatment), treatment should be discontinued immediately and spiramycin should not be used thereafter, even if alone or in combination. Rodogyl therapy should be discontinued if patients experience symptoms of ataxia, dizziness or confusion. Since Rodogyl contains metronidazole, the risk of neurological exacerbation should be considered in patients with severe, chronic, or progressive central or peripheral nervous system disease. Spiramycin is not recommended in patients with glucose-6-phosphate-dehydrogenase deficiency. Rare cases of hemolytic anemia have been reported in these patient populations. In patients with a history of hematologic disorders and in patients receiving high doses and/or prolonged therapy, a complete blood count should be performed frequently. In the case of leukopenia, the continuation or discontinuation of rodogyl therapy depends on the severity of the infection. In cases where prolonged Rodogyl therapy is required, monitoring for signs suggestive of central or peripheral nervous system adverse effects such as paresthesias, ataxia, dizziness, confusion, or convulsions should be monitored. Several cases of increased oral anticoagulant activity have been reported in patients receiving Rodogyl antibiotics. The degree of infection, age, and general condition of the patient may be risk factors for this condition. In these cases, it is difficult to determine whether the infection or the treatment of the infection affects the INR. However, there are several classes of antibiotics commonly associated with this problem, particularly fluoroquinolones, macrolides, tetracyclines, cotrimoxazole, and some cephalosporin antibiotics. Driving and using machines: Patients should be warned about the risk of dizziness, confusion, or hallucinations while taking Rodogyl and advised not to drive or operate machinery if these disturbances occur. Pregnancy: Clinically, several studies analyzing pregnancies exposed to metronidazole have shown no teratogenic or fetotoxic effects. Animal studies have not revealed evidence of a teratogenic effect of metronidazole. However, more epidemiological studies are needed to draw firm conclusions. Therefore, pregnant women use the drug only when prescribed by a doctor and weigh the benefits. For spiramycin, the use of spiramycin may be considered in pregnant women. The use of spiramycin during pregnancy has not been shown to have teratogenic or fetotoxic effects. In general, the drug can be used during pregnancy in cases where it is absolutely necessary. Lactation: spiramycin and metronidazole are excreted in breast milk. Therefore, Rodogyl should not be used while breastfeeding. Overdose: There is currently no specific antidote for spiramycin and metronidazole. If an overdose of Rodogyl occurs, mainly symptomatic and supportive treatment. When spiramycin overdose, patients may experience gastrointestinal symptoms such as nausea, vomiting, diarrhea. Some cases of patient prolongation of the QT interval, which may be reduced upon discontinuation of therapy, have been observed in neonates treated with high doses of spiramycin and following intravenous administration of spiramycin in subjects at risk for prolongation of the QT interval. QT interval length. Therefore, if an overdose of spiramycin occurs, the QT interval should be measured, especially in the presence of other risk factors (hypokalemia, congenital QT prolongation, in combination with drugs that prolong the QT interval). . With regard to metronidazole, cases of single doses up to 12 g have been reported with only vomiting, ataxia and mild disorientation. In summary, Rodogyl has two main ingredients, spiramycin with a concentration of 750,000 IU and metronidazole 125mg. This is a combination antibiotic commonly used in the treatment and prevention of oral infections. The drug can cause some unwanted effects, mainly on the digestive tract. Patients need to follow the correct instructions and dosage to ensure safety and effectiveness when using the drug.
Follow Vinmec International General Hospital website to get more health, nutrition and beauty information to protect the health of yourself and your loved ones in your family.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.