This is an automatically translated article.
Posted by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General HospitalCrohn's disease is an inflammatory bowel disease with symptoms of abdominal pain, severe diarrhea, fatigue, weight loss, and malnutrition. The inflammation caused by Crohn's disease often spreads deep into the layers of intestinal tissue, which is both painful and debilitating, and can sometimes lead to life-threatening complications.
Although there is no cure for Crohn's disease, care and treatment can significantly reduce the signs and symptoms of the disease and even bring about long-term remission.
1. Causes of Crohn's Disease
Currently, the exact cause of Crohn's disease is unknown. Previously, it was suspected that diet and stress led to the disease, but now doctors say these factors aggravate the condition but are not the cause of Crohn's disease. Certain factors, such as genetics and having an immune system problem, play a role in the development of Crohn's disease.
Immune System . It is theorized that certain viruses or bacteria trigger Crohn's disease. When the patient's immune system tries to fight off the invading microorganism, an abnormal immune response occurs, causing the immune system to mistakenly attack not only the invading organism but also the cells. in the digestive tract. Genetic. Crohn's disease is common in people who have a family member with the disease, so genes may play a role in making future generations more likely to develop the disease than other families.
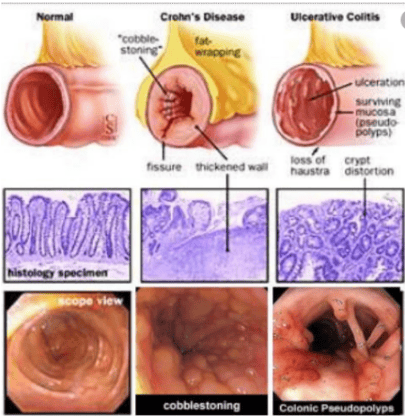
2. Difficulty in treating Crohn's disease in pregnant women
Drug use counseling for patients during pregnancy is still difficult due to lack of reliable data to base decisions on as well as limitations in labeling about drug use during pregnancy. . The use of drugs during pregnancy is quite common and is increasing as the age of pregnant women tends to increase. Pharmacists should carefully evaluate the potential risks of drug use against the risks if the condition is left untreated during pregnancy. When counseling patients, pharmacists should provide information regarding the benefits and risks of drug use, so that the mother can make the best decision for herself and her unborn baby.
According to a survey from 2006 to 2008, over 90% of women took at least one prescription or over-the-counter (OTC) medication during pregnancy. 70% of people surveyed used at least one prescription drug during this period. From 1976 to 2008, the number of women using prescription drugs during pregnancy more than doubled. If only in the first 3 months of pregnancy, this number increases more than 3 times. That shows that the use of drugs during pregnancy is common and tends to increase over time. Furthermore, the percentage of pregnant women exposed to drugs that can cause birth defects is 6%; in which, 3% of babies are born with physical or mental birth defects.
In December 2014, the US Food and Drug Administration (US FDA) issued a drug labeling regulation for pregnant and lactating women (PLLR) to address limitations in prescription drug labeling. Before the issuance of the PLLR, drugs were divided into categories A, B, C, D and X; where A is considered safe and X is likely to cause birth defects. This classification is trusted by healthcare professionals, but is often misunderstood and abused because it does not accurately represent differences in risk levels and does not provide clinically relevant information. Results from a review showed that only 4 of 172 drugs (2.3%) approved by the US FDA between 2000 and 2010 had sufficient data to determine a teratogenic risk while more than 70% of drugs (126/172 drugs) there are no human data to determine this risk. The final regulation, which took effect on June 30, 2015 required the removal of information about the safety classification during pregnancy on drug labels. This rule does not apply to over-the-counter drugs. The PLLR also incorporated changes in labeling to provide a unified framework for providing information about the risks and benefits of drug use during pregnancy and lactation, as well as its use. drugs in women and men of reproductive age.
3. Changes in the pharmacokinetics and pharmacodynamics of drugs during pregnancy
Safety of drug use is often focused on the first 3 months of pregnancy because the drug often works on an "all or not" principle (stillbirth) in the first 8 weeks and most allergies Birth defects occur early in development. Changes in the physiological function of the organ systems in the mother's body begin to take place in the first 3 months of pregnancy and peak in the second 3 months of pregnancy.
Changes in cardiovascular, respiratory, gastrointestinal, renal and hepatic function during pregnancy may lead to changes in the pharmacokinetics and metabolism of the drug. In addition, changes in the transport protein and enzyme systems further alter drug metabolism during pregnancy. A recent systematic review found a large gap from knowledge of pharmacokinetic changes during pregnancy to understanding the clinical effects of these changes in mother and child. The teratogenic risk of the drug should be weighed according to the gestational age of the fetus. The risk of drug effects on the fetus is highest at the corresponding stage of fetal development (eg, drugs that affect cartilage growth during the period of skeletal development should be avoided).
Absorption: Increased gastric pH during pregnancy may affect the absorption of drugs of a weak acid and weak base. In addition, nausea, vomiting, and delayed gastric emptying may also alter the absorption of the drug.
Distribution: Due to increased maternal body fat during pregnancy, the volume of distribution of lipophilic drugs may be increased. The volume of distribution of drugs that are highly bound to plasma proteins is increased by decreased albumin concentrations. Unbound drug concentrations remain relatively stable because these drugs are rapidly eliminated by the liver and kidneys. Lipid-loving drugs have a reduced rate of elimination due to their larger volume of distribution.
Metabolism: Changes in CYP450 enzymes affect drug metabolism during pregnancy. The concentrations of the enzymes CYP3A4 and CYP2D6 were both increased, while that of CYP1A2 decreased. Studies have also documented changes in the enzymes uridine 5'-diphosphate glucuronosyltransferase and N-acetyltransferase. In addition, increased levels of estrogen and progesterone also change the activity of liver enzymes, which can increase the elimination of some drugs but cause accumulation of others.
Elimination: During pregnancy, maternal plasma volume, cardiac output, and glomerular filtration rate increase, which may lead to decreased concentrations of drugs eliminated by the kidneys. In general, the body's exposure to the drug during pregnancy is reduced due to increased elimination of the drug in both conjugated and unbound forms. This may be beneficial in reducing adverse pregnancy events.

4. Using drugs during pregnancy: safe and harmful
Studies using the drug in pregnancy often produce results that tend to be “skewed against the null hypothesis,” meaning that the drug is often thought to be more likely to cause birth defects than not to work. adverse effects on the fetus. Pregnant women are also more likely to report adverse outcomes (such as birth defects) than to give birth to healthy babies. The literature also shows that studies that found a drug that did not increase the risk of adverse events are often less published or receive less media attention. Pharmacists should be aware of these limitations and consider providing information to patients about these limitations. The medications presented in this article focus on the latest evidence and problems women may experience during pregnancy that pharmacists may be less aware of than other common pregnancy conditions. period, like nausea.
5. Treatment of Crohn's disease in pregnant women
The impact of IBD conditions on pregnant women depends on how active the disease is during pregnancy. The recurrence rate for Crohn's patients during pregnancy according to one study is about 20%. If the disease is active at the time of pregnancy, up to two-thirds of patients will continue to have disease or worsen during pregnancy and the risk of preterm birth, birth Out with low weight is also higher. However, if at the time of pregnancy, the patient is in a stable stage, without disease progression, the risk to the fetus is not different from that of normal pregnant women. Therefore, the advice for patients with Crohn's disease is to achieve and maintain a stable disease stage if they want to become pregnant.

6. Drugs used during pregnancy to treat Crohn's disease
According to the FDA, among the drugs used to treat Crohn's, methotrexate belongs to group X and is contraindicated in both pregnancy and lactation. In addition, because methotrexate can stay in the body for a long time, patients need to wait 3-6 months after stopping treatment before becoming pregnant. The group of immunomodulatory drugs including azathioprine and 6-mercaptopurine of group D can cause abnormalities and abnormalities in the fetus. The other drugs are mainly in groups B and C.
Aminosalicylate group: Sulfasalazine has undesirable effects causing folic acid reduction, so it is necessary to supplement folic acid 1mg x 2 times/day. Mesalamine is safe for breastfeeding women. Sulfasalazine is excreted in milk but is also safe for nursing women. • Antibiotics: If you must use antibiotics during pregnancy, you can choose amoxicillin-clavulanic acid. During breast-feeding, amoxicillin-clavulanic acid or ciprofloxacin can be used. • Corticosteroids: During the first trimester, corticosteroid use may increase cleft palate. Breastfeeding women can use prednisone and prednisolone. Data on budesonide are currently unknown. • Probiotics: Probiotic drugs are all group B for pregnant women and so far the main controversial issue is when to stop these drugs. Infliximab does not cross the placenta until 20 weeks. With the exception of certolizumab, biologics should be discontinued in the third trimester of pregnancy. However, in high-risk patients with advanced disease, the drug may still need to be used throughout pregnancy. Vinmec International General Hospital is a prestigious address trusted by many patients in performing diagnostic techniques for digestive diseases, diseases that cause chronic diarrhea, Crohn's disease... Along with that, at Hospital At Vinmec Hospital, screening for gastric cancer and gastric polyps was performed through gastroscopy with an Olympus CV 190 endoscope, with NBI (Narrow Banding Imaging) function for results. The imaging results of mucosal pathology analysis are clearer than conventional endoscopy, detecting ulcerative colitis lesions, early gastrointestinal cancer lesions... Vinmec Hospital with facilities With modern equipment and a team of experienced specialists, always dedicated to medical examination and treatment, customers can rest assured with gastroscopy and esophagoscopy services at Vinmec International General Hospital.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
ReferencesDao Van Long, Dao Viet Hang. Autoimmune allergic disease of the gastrointestinal tract. Medical Publisher. Bernstein C. N. and Shanahan F. (2008). Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut,57(9), 1185 - 1191. 3. Nell S., Suerbaum S., and Josenhans C. (2010). The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol, 8(8),564-577. 4. Binder V. (1998). Genetic epidemiology in inflammatory bowel disease. Dig Dis Basel Switz, 16(6), 351-355. 5. Montgomery S. M., Morris D. L., Pounder R. E. et al (1999). Asian ethnic origin and the risk of inflammatory bowel disease. Eur J Gastroenterol Hepatol, 11(5),543-546.