This is an automatically translated article.
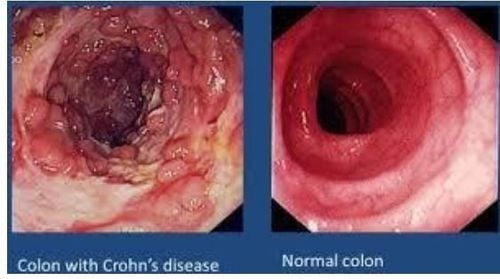
Posted by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General HospitalIn May 2018, the US Food & Drug Administration approved the use of tofacitinib for the treatment of moderate to severe ulcerative colitis (UC). The drug tofacitinib represents the first Janus kinase (JAK) inhibitor approved in inflammatory bowel disease (IBD). This article will provide a practical approach to clinical use of tofacitinib, as well as updates on other JAK inhibitors currently in development.
1. The drug tofacitinib represents a Janus kinase (JAK) inhibitor.
1.1 Results of some studies Janus kinase (JAK) inhibitors are important in intracellular signaling, they are considered to be one of the important bridges between cytokines that activate receptors on the cell surface and transcription of genes in the nucleus.Each cytokine receptor is associated with 2 JAK molecules. When a cytokine finds its receptor, the associated JAKs are activated. These activated molecules, in turn, phosphorylate receptors to bind to signal transducers and activators of transcription proteins (STATs), and then migrate to the cell nucleus to activate new gene transcription. .
In the pivotal induction trials of tofacitinib in moderate to severe ulcerative colitis, 2 trials OCTAVE1 and OCTAVE2 were performed in more than 1100 patients. They were randomized in a 4:1 ratio to receive tofacitinib 10 mg or placebo twice daily. The primary endpoint of the study was clinical remission of symptoms by week 8. Outcomes were defined as a total Mayo score less than or equal to 2, with no subscore greater than 1, and a bleeding score rectal score was 0. In OCTAVE1, 18.5% of tofacitinib-treated patients achieved the endpoint and 8.2% of placebo-treated patients (p=0.007). In the OCTAVE2 trial, the clinical remission rate at week 8 was 16.6% in tofacitinib-treated patients and 3.6% in placebo-treated patients (p < 0.001). Mucosal healing was defined as a Mayo endoscopic sub-score of 0 or 1, which was achieved after 8 weeks in 28.31% of tofacitinib-treated patients, 12-16% higher than in patients were treated with placebo (p < 0.001 for both trials).
1.2 Advantages of tofacitinib One notable property of tofacitinib is its ability to initiate action quickly. A post hoc analysis of induction trials examined patient-reported outcomes at the level based on his or her symptom diary. The differences in rectal bleeding rates and stool counts in tofacitinib-treated people compared with placebo were seen as early as 3 days after starting the drug.
Patients who completed the induction trial and had a clinical response were then randomized to treatment in the OCTAVE Sustain maintenance trial up to 5 mg twice daily, 10 mg twice daily, or placebo for 52 weeks. The primary goal was clinical remission at 52 weeks. This was achieved in 41% of patients on 10 mg twice daily, 34% on 5 mg twice daily, and only 11% in users. placebo (p < 0.001 for both comparisons). This difference was clinically and statistically significant between the 2 doses of tofacitinib and placebo.
1.3 Role of high dose tofacitinib in acute severe ulcerative colitis A series of studies from the University of Michigan explored the potential role of high dose tofacitinib in the treatment of acute severe ulcerative colitis. Doses of 10 mg 3 times/day for 3 days have been used in conjunction with the patient's usual treatments. The results showed that, 3 out of 4 patients saw significant improvement; however, 2 of these 3 patients underwent elective resection for multifocal dysplasia. Therefore, the role of tofacitinib in acute severe ulcerative colitis remains unclear.
1.4 Side effects of tofacitinib The use of tofacitinib to treat ulcerative colitis can potentially lead to serious infections and malignancies in patients.
In induction trials of ulcerative colitis have shown a higher rate of infection in patients treated with tofacitinib. In the maintenance trial of ulcerative colitis, rates of mild to serious adverse events and infections were similar between tofacitinib and placebo.
1.6 How tofacitinib is used in clinical practice Due to FDA restrictions on the indications for tofacitinib, physicians have stopped using the drug in biologic-nave ulcerative colitis patients. However, in a 2018 meta-analysis of randomized trials in patients with ulcerative colitis exposed to biologics, tofacitinib emerged with the strongest therapeutic effect in relation to clinical remission (OR). , 12.6; 95% CI, 2-5-64), induced mucosal healing (OR, 4.7; 95% CI, 2.2-9.9). Not only is it potent and fast acting, tofacitinib is also an ideal agent for patients with inflammatory bowel disease (IBD).
When advising patients on safety, the physician must report a 5%-6% risk of herpes zoster if they continue to take 10 mg of BID for 1 year. Of course, with the recombinant zoster vaccine, this risk can be significantly reduced. Patients are also advised to have blood counts, liver biochemistry and lipid profiles checked every 3 months, during the course of medication.
2. Update on Other Janus Kinase Inhibitors in Inflammatory Bowel Disease (IBD)
2.1 Role of filgotinib in the treatment of inflammatory bowel disease There are a number of selective JAK1 agents currently being developed for the treatment of inflammatory bowel disease (IBD). In theory, selective JAK1 antagonists could "widen the therapeutic window", allowing higher doses to be used to achieve efficacy without compromising safety.
Study results showed adverse events occurred in 18% of the opiate group and 9% of the placebo group.
2.2 Role of upadacitinib in the treatment of ulcerative colitis Preliminary results of the U-ACHIEVE phase II study of upadacitinib in ulcerative colitis were presented recently. A total of 250 patients enrolled in the study, more than three-quarters of which were biologics, were randomized to four different doses of the once-daily extended-release (ER) formulation of upadacitinib or placebo. (Based on pharmacokinetic studies, 15 mg of ER is thought to be equivalent to 6 mg of immediate-release BID [IR] and 30 mg of ER is said to be equivalent to 12 mg of IR BID.) The primary endpoint was remission. clinical reduction as adjusted for Mayo-adjusted score (no physician global assessment) at week 8. This endpoint was met for doses of 15 mg, 30 mg, and 45 mg (14.3, respectively). %, 13.5% and 19.6% vs 0% placebo; p < 0.05 for all 3 doses). The primary endpoint of endoscopic improvement (Mayo endoscopic score, 0-1) was significant for all 4 doses of upadacitinib, ranging from 14.9% (7.5 mg) daily to 35.7% (45 mg) per day, compared with 2.2% placebo (p < 0.05 for all).
Side effects of upadacitinib:
The rate of serious adverse events occurred highest in placebo-treated patients. Serious infections ranged from 0-3.6% in patients treated with upadacitinib and 4.3% in the placebo group. Only one case of herpes zoster was identified during treatment with upadacitinib. Histological remission endpoint (Geboes score <2) was significant for the 3 higher doses of upadacitinib. The endpoint of "mucosal healing" combined with endoscopy and histological improvement was also significant for multiple doses of upadacitinib. In summary, JAK inhibition represents a potent, rapidly effective mechanism for reducing inflammation in inflammatory bowel disease (IBD). Tofacitinib is approved for the treatment of moderate to severe ulcerative colitis in patients who have failed anti-TNF therapy. Selective JAK1 inhibitors appear to be effective in moderate to severe Crohn's disease and upadacitinib is effective in ulcerative colitis.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
ReferencesO'Shea JJ, Plenge R. JAKs and STATs in immunoregulation and immune-mediated disease. Immunity 2012;36:542-50. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723-36. Hanauer SB, Panaccione R, Danese S, et al. Tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2019;17:139-47. Edward V. Loftus, A Practical Approach to JAK Inhibitors for Inflammatory Bowel Disease in 2020, practicalgastro. May 2020 • Volume XLIV, Issue 5