This is an automatically translated article.
The article was written by MSc Nguyen Thi Hang - Laboratory Doctor, Laboratory Department - Vinmec Times City International General HospitalIn respiratory diseases, pleural effusion (TDMP) is a common syndrome and has many causes. Correct diagnosis of the cause of TDMP is difficult but essential for the treatment and prognosis of the disease.
In recent years, world medicine has made remarkable progress to diagnose the etiology of endocarditis such as open pleural biopsy (STMP), blind biopsy, endoscopic pleural biopsy... but The method of cytology of pleural fluid (DMP) is a leading important diagnostic method with many advantages of simplicity, ease of implementation and can be applied in all laboratories at the same time for results. fast and high accuracy.
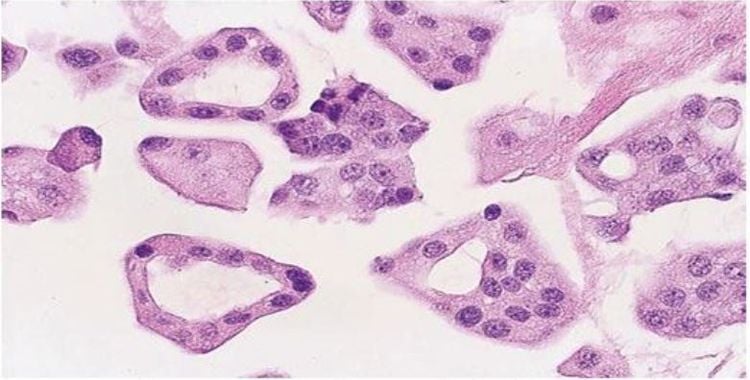
However, correctly determining whether cells in the fluid are malignant or reactive mesothelial are problematic for routine cell smear cytology (CS). Diagnosis of CS has low sensitivity because cells are often overlapped or lost during the test.
1. What is cell block technology (CB)?
Cell block technique (CB) is a valuable method in the diagnosis of fluid in body cavities. To increase sensitivity, most laboratories use two or more diagnostic methods. With the smear alone, about 67% of the malignant solutions were identified. If combined with centrifugation, filtration and CB, about 83% is determined.
In the study of some authors around the world, the sensitivity and specificity of CB up to 90% (1). Nythzamanda Nathan et al., when studying the value of CB on 1009 cases of fine needle aspiration and membrane translocation, the sensitivity of the cell mass was 89.4% (2). CB DMP technique helps diagnose 15% more malignant TH compared with CS technique in the study of Udasimath S. (3). The correct diagnostic value of CB is higher than that of CS due to better preservation of tissue fragments and special staining, especially immunohistochemistry (HMMD) because many criteria can be prepared. copies are the same, the background is limited and the control template can be standardized (4).
In the study of Nguyen Thi Hang et al. (2012) (5), diagnosing schizophrenia by CB technique has a significantly higher sensitivity than blind schizophrenia (86.7% and 66.7%). This result is consistent with the study of Edmund Cibas and Cs (2009), fluid cytology (CB and CS) is more sensitive than blind biopsy in detecting malignant lesions of the serosa cavity (71%). compared to 45%) (1) . According to Claire W. Michael et al (2012) (5), the diagnostic value of malignancy of TBH ranges from 62 to 90%. Therefore, when cytology fails to diagnose, perform pleural endoscopy and biopsy, without blind STMP because this method only improves 7% of negative cases.
Trắc nghiệm: Làm thế nào để có một lá phổi khỏe mạnh?
Để nhận biết phổi của bạn có thật sự khỏe mạnh hay không và làm cách nào để có một lá phổi khỏe mạnh, bạn có thể thực hiện bài trắc nghiệm sau đây.2. Method of pleural fluid cell block
The pleural effusion method is a simple, inexpensive method and does not require any special equipment or techniques. Multiple fragments from the candelabra can be extracted for specific staining and HMMD to identify the origin of metastatic tumors with high sensitivity and specificity. However, the limitation of the method is that the time for diagnosis is long (3 - 4 days) compared with the smear smear method (1/2 - 1 day).
This technique is now recommended for routine application in cytology-pathology laboratories along with smear method and applied to all membrane fluids. Facilities that do not yet have HMMD testing may send candle blocks to qualified laboratories for staining as needed.
3. Technical principles
To concentrate single, scattered cells in the fluid into a mass that can be transferred, cast, cut, and stained like conventional histological procedures.Preparation
Implementation staff Technician
Means, chemicals Glass jars with lids, volume 250 ml. Glass test tubes with size 10 x 1.6 cm or centrifuge tubes of 50ml volume Heparin (solution type) Cytorich Red, Mucolex Formol neutral 10% Centrifuge, cassette, slide, histological wrapper ( non-stick type) Small wooden stick, cotton wool (waterproof) Instruments, chemicals of routine histology (H&E, PAS staining) Patient Patient has effusion of body cavities (membrane) heart, pleura, peritoneum, cerebrospinal fluid ...), bronchial lavage fluid, urine, fluid from cysts, joint fluid...
Test sheet Filled in all administrative information of the patient ( name, age, bed number, room number, ward), clinical diagnosis, summary of clinical and laboratory information, request for fluid cell block test; date and time of collection, general comments (color, amount of fluid, blood, mucus...).
4. Steps to take
4.1 Specimen collection 250ml glass vial is evenly coated with 1000 units of heparin on the sides and bottom before pouring. Heparin prevents blood from clotting, so it doesn't trap cells in a blood clot.
Translations are taken at clinical departments. Take the solution into a glass jar. The amount of fluid depends on the patient but should be taken from 50 to 250 ml to get many samples (if patient conditions allow).
Close the lid, label (name, age, department, clinical diagnosis) and immediately send it to the Pathology laboratory. If it is not possible to send immediately, store the specimens in the lower compartment of the refrigerator at 40C. Specimens are stored for no more than 2 weeks.
4.2 Carry out the technique In the Pathology - Cytology room, the technicians will conduct a general assessment of the color, the amount of fluid, the presence/absence of a lot of blood or mucus.
If the fluid has a lot of blood, give 1ml of Cytorich red/50ml of solution, if the solution has a lot of mucus, add 1ml of Mucolexx/50ml of solution, shake well and leave for about 5 minutes to dissolve the mucus.
If you have a 50ml large tube centrifuge, proceed to step 1.
If a large tube centrifuge is not available, leave the vial for 8-10 hours to allow the cells to settle down, then remove the surface layer, shake the cell residue well, and then divide into test tubes. small (1.6 x 10cm), tightly stopper with waterproof cotton, then proceed from step 1.
Note: If the fluid has been preserved in clinical departments for 8-10 hours, cells have settled below then proceed to perform the technique (as described above).
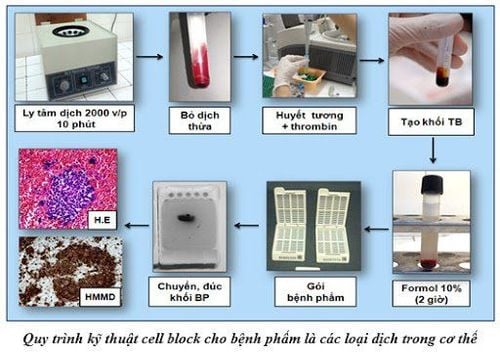
Step 1: Put the test tubes containing the solution into the centrifuge for 10 minutes at 2000 rpm.
Step 2: Remove the upper clear layer to remove the cell sediment and then fix the cell residue in 10% formol in a 60oC incubator for about 2 hours.
Step 3: Centrifuge the specimen again at 2000 rpm for 10 minutes if necessary (if the specimen has formed a solid mass, the procedure can be switched directly from step 2 to step 4).
Step 4: Remove the formol into the waste water container. Use a small wooden stick to remove the mass of cells from the tube and place on a piece of paper (non-stick paper available in the histology laboratory). Wrap the cell mass in this paper and place it in a cassette labeled with the patient's name and cell block number.
Step 5: Inject the cassette with the cell mass into the routine histological procedure.
Step 6: The cut pieces from the cell mass are then stained with Hematoxylin - Eosin (H&E) and special staining (Periodic acid - Schiff (PAS), mucicarmine...) and immunohistochemical staining when needed. Slices are usually 3–5 μm thick.

5. Evaluation of results
Reviewer: Doctor of Pathology - Cytology.
Cell masses preserve tissue structures better than cell smears, allowing for multiple excision of the same slides, H&E, special staining, and immunohistochemical staining for diagnosis Diagnosis can identify many types of lesions such as fungi, malignant lesions or direct the origin of the primary tumor.
6. Errors and handling
During the technical process, patients may be confused due to the multi-step process, the test tubes are broken, and the specimens are lost... Need to work in a focused way, always have checks and contrasts to avoid unnecessary errors.Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
Source Reference:Cytology: Diagnostic Principle and Clinical Correlates. Edmund S. Cibas, Barbara S. Ducatman, eds. Philadelphia, USA. : Sauders Elsevia, The Third Edition ed. 2009,.. "Improved preparation and its Efficacy in Diagnostic Cytology",. Nythyananda A. Nathan, Eddie Narayan, BApp Sci, et al. s.l. : American Journal of Clinical Pathology, 2000, Vols. 114, pp. 599 - 606. Comparative study of conventional smears and cell blocks in the cytodiagnosis of serous effusions. Udasimath S, Surekha U. Arakeri. s.l. : Pathology., 2008. Diagnostic accuracy of cell-block or tissue-fragment histology and cytology by fine needle lung aspiration. Chulin Wu, Zeng Y, Wu P, et al. s.l. : Chinese journal of pathology, 2002. Cytological diagnosis of pleural fluid by cell mass technique. Nguyen Thi Hang, Nguyen Thu Huong, Vu Hoang Anh, Dang Van Duong, Vu Manh Thang. Hanoi : Vietnam Journal of Clinical Medicine, 2012. Serous effusions: Etiology, Diagnosis, Prognosis and Therapy. Claire W. Michael, Davidson B, and Firat P,. London. : Springer, Editor. , 2012.